Abstract
Introduction
This study aimed to compare treatment satisfaction with two dosing regimens (two teriparatide [TPTD] self-injection systems) in osteoporosis patients at high risk of fracture.
Materials and methods
In this open-label crossover randomized trial comparing self-injected once-daily (1/D)-TPTD with self-injected twice-weekly (2/W)-TPTD, three satisfaction variables were evaluated by questionnaire for 2 years. The primary endpoint was overall satisfaction and secondary endpoints were satisfaction with treatment effectiveness and with utility of the self-injection device. Changes in quality of life (QOL) assessed by EuroQol-5 Dimension, pain assessed by visual analogue scale (VAS), and anthropometric parameters were also analyzed. Safety was evaluated based on the incidence and severity of adverse events (AEs).
Results
The 1/D-TPTD and 2/W-TPTD groups consisted of 180 (75.9 ± 7.3 years) and 179 (age: 75.5 ± 6.9 years) patients, respectively. After 26 weeks of treatment, no significant between-group difference in the persistence rate (79.4% vs 72.6% in the 1/D-TPTD and 2/W-TPTD groups, respectively), distributions of overall satisfaction scores, and satisfaction with treatment (p > 0.05) were observed. However, several items of satisfaction with the utility of the injection device were significantly higher in the 2/W-TPTD group (p < 0.05). Statistical improvements from baseline values were observed in QOL and pain VAS in both groups (p < 0.05). No serious AEs were reported.
Conclusion
The between-group similarity of overall treatment satisfaction and effectiveness scores and between-group difference in satisfaction with the utility of the self-injection device was useful information for real-world treatment of osteoporosis. Both medication regimens were well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a chronic disorder characterized by low bone mass and disordered skeletal microarchitecture, resulting in impaired bone strength and an increased risk of fragility fracture [1].
Several pharmacological agents are available to lower fracture risk, either by reducing bone resorption or by stimulating bone formation [2]. Recent studies suggest that bone anabolic agents have important roles in the initial treatment of patients with osteoporosis, especially for those at very high risk of fracture [3]. Moreover, it was reported that bone mineral density (BMD) accrual is maximized when patients are given anabolic agents first, followed by potent antiresorptive therapy [4].
Nonadherence to pharmacological agents in osteoporosis is a well-recognized problem. Treatment discontinuation due to poor adherence places an enormous burden on patients by increasing rates of fractures and use of healthcare resources [5, 6]. Actually, a meta-analysis of existing studies reported that good adherence, compared with nonadherence, significantly reduced the risk of all fractures by 28%, the risk of hip fracture by 49%, and the risk of non-vertebral fracture by 26% [7].
Extending the dosing interval of bone resorption inhibitor improves medication adherence [8]. The extension of dosing intervals may be one element contributing to improvement in therapeutic adherence. Other elements may be improved patient education, enhanced healthcare provider–patient interaction, taking into account patient's preferences, and involving them in treatment decisions [5].
In Japan, the once-daily [9], once-weekly [10], and twice-weekly [11] teriparatide dosing regimens have similar efficacy and have been approved for the clinical treatment of high-risk osteoporosis. Two of these regimens, the once-daily and twice-weekly, involve the use of self-injectable formulations and are widely used. The self-injection device used to deliver the twice-weekly formulation has an invisible needle that does not require replacement, and contains a much lower total dose of teriparatide than used in the once-daily formulation. These benefits are expected to lower the number of needle stick accidents and reduce self-administration complexity [12, 13], thereby improving patients’ satisfaction and adherence.
Although patient satisfaction with the osteoporosis antiresorptive agents, denosumab and bisphosphonates, has been studied in a crossover manner [14, 15], differences in treatment satisfaction between formulations of teriparatide have not been compared in a similar manner.
The primary objective of this study was to compare once-daily and twice-weekly self-injectable formulations of teriparatide by evaluating patient satisfaction (including both adherence and persistence), efficacy, and safety in a prospective, open-label, crossover, randomized trial. This report describes the results from the first 26 weeks, which includes the primary endpoint of the study.
Materials and methods
Ethical approval
All procedures performed in studies involving human participants were in accordance with institutional and/or national research committee ethical standards and with the ethical principles set out in the Declaration of Helsinki and its amendments or comparable ethical standards. The study was also conducted in compliance with the Clinical Trials Act and related ministerial orders, and all applicable regulations and ethical requirements. The study protocol was approved by the Toranomon Hospital Certified Review Board. Written informed consent was obtained from all individual participants prior to inclusion in the study.
Study subjects
This multicenter open-label crossover study (jRCTs031210187) planned to enroll 400 postmenopausal women with primary osteoporosis [16], aged 60 years or older. Patients at high fracture risk were eligible if they satisfied any of the following inclusion criteria: BMD < 60% of the young adult mean (YAM) or < − 3.3 standard deviation (SD); ≥ 2 vertebral fractures (assessed by a semi-quantitative method [17]) between the fourth thoracic vertebra (Th4) and fourth lumbar vertebra (L4); a grade 3 vertebral fracture; ≥ 1 spinal vertebral fracture at Th4–L4 and BMD ≤ -2.5 SD of YAM; or history of hip fracture. Patients were excluded if they had (1) a diagnosis of secondary osteoporosis; (2) bone loss induced by diseases other than osteoporosis; (3) hypersensitivity; (4) any contraindication to teriparatide; (5) severe hepatic failure, renal failure, or cardiac failure; (6) inability to self-administer the drug; (7) experience with using any auto-injection device; (8) dementia when medical interview of the patient was difficult to conduct; (9) receipt of an investigational trial anti-osteoporosis drug within the 52 weeks prior to giving informed consent; (10) been hospitalized; (11) a history of teriparatide treatment.
Study design
After providing their informed, written consent to participate, eligible subjects were randomly divided into two groups. One group received a once-daily dose (20 μg self-injection) of teriparatide (1/D-TPTD group) and the other, a twice-weekly dose (28.2 μg self-injection) of teriparatide (2/W-TPTD group) for 26 weeks (the first quarter of the 104-week study period). The dosing regimen was then switched and treatment was continued for another 26 weeks (one half of the total study period). After completion of the 52-week crossover study, patients were allowed to receive either a daily dose or twice-weekly dosing depending on their preference, and the treatment continued for another 52 weeks (ending the study). All patients received daily vitamin D supplementation (25 μg/day) throughout the study period.
There was no basis for setting the number of cases to statistically test the primary outcome hypothesis. Therefore, in consultation with other researchers, we determined that a group difference of at least 1 point would be a clinically meaningful and large difference, and assumed a group difference of 0.7 to 1.0. We further assumed a score variability of SD 1.4 to 2.4 and set the number of cases per group at 200.
Endpoints
Patient satisfaction was investigated using the Patient Satisfaction Questionnaire (Online Resource 1). The questionnaire consists of one question on overall satisfaction (asked at 26 weeks), two questions on the effectiveness of treatment (asked at 26 weeks), and 12 questions on the utility of self-injection device (asked at 2, 4, 13, and 26 weeks) with ease of use rated from difficult to easy on a 3- or 6-point scale. The primary endpoint of the study was the degree of overall patient satisfaction at 26 weeks. The secondary endpoints were (1) overall patient satisfaction at 52 and 104 weeks, (2) patient satisfaction with treatment at 26, 52, and 104 weeks, (3) time course of patient satisfaction with the device utility (12 questions), (4) preference when the patient was allowed to choose at 52 weeks, (5) adherence, and (6) efficacy of the treatment. Adherence was judged to be good if self-injection rate was ≥ 80%, self-injection rate was ≥ 50% during the final 4 weeks, and attendance at a final visit was within the predetermined time limit. Treatment efficacy was judged by the number of incident vertebral or non-vertebral clinical fractures, change of BMD, quality of life (QOL) on the EuroQol-5 Dimension (EQ-5D) scale, pain evaluated on a visual analogue scale (VAS), and parameters of bone structure analysis. Clinical fractures were identified through monitoring clinical symptoms and confirmed with radiography by the physicians. BMDs were measured at L1–L4 or L2–L4, the femoral neck, and total hip by dual energy X-ray absorptiometry. Safety of treatment was evaluated based on incidence, type, and severity of adverse events (AEs). AEs were evaluated by the attending physician through an interview when the patient visited the institute.
Statistical analyses
Endpoints were analyzed in the full analysis set (FAS). All data are presented as mean ± SD or number and percentage. The differences in baseline characteristics between the 1/D-TPTD and 2/W-TPTD groups were evaluated by the t-test and Fisher χ2 test used for continuous variables and categorical variables, respectively. The difference between the average score for the primary endpoint was compared using the t test. Also, the differences in score distribution between groups were evaluated using the Cochran-Mantel–Haenszel test. Other patient satisfaction endpoints were also analyzed as continuous variables using the t test. The paired t test was used to assess time-dependent changes in QOL, VAS, and anthropometric parameters from baseline. Also, incidences of clinical fractures were compared using the Fisher exact test. Differences with p values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
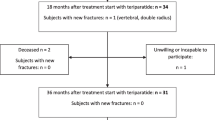
A total of 381 patients (190 in the 1/D-TPTD group and 191 in the 2/W-TPTD group) were enrolled between July 2021 and September 2022. Twenty patients were excluded from analysis mainly due to canceled participation prior to the start of treatment and the remaining 359 patients, 180 and 179 for the 1/D-TPTD and 2/W-TPTD group, respectively, served as the FAS population.
Patient characteristics
Table 1 summarizes patient characteristics. The mean and standard deviation of age at the time of registration was 75.9 ± 7.3 years in the 1/D-TPTD group and 75.5 ± 6.9 years in the 2/W-TPTD group. Approximately 31% of patients in each group had a clinical fracture within 1 month before randomization. No significant differences in those listed characteristics were observed between the two groups (p > 0.05). The most common anti-osteoporosis drugs used prior to the start of TPTD were bisphosphonates (18.4%), followed by eldecalcitol (16.4%).
Persistence
During the 26-week observation period, 37 patients in the 1/D-TPTD group and 49 patients in the 2/W-TPTD group stopped the treatment, and thus the continuation rate at the end of 26 weeks was 79.4% and 72.6%, respectively (p = 0.671).
Endpoints
The degree of overall patient satisfaction, the primary endpoint of the study, is summarized in Table 2. The distributions of overall satisfaction scores in both groups were similar and no significant difference was observed between the groups (p = 0.522). The overall satisfaction score, treated as a continuous variable, was 3.5 ± 1.3 in the 1/D-TPTD group and 3.4 ± 1.2 in the 2/W-TPTD group and not significantly different between the groups (p = 0.523). The scores of patient satisfaction with the effectiveness of treatment at 26 weeks were also similar in both groups, and no significant difference was observed (p > 0.05, Table 2).
Scores of patient satisfaction with the utility of the injection device (12 questions each) and the overall scores are summarized in Online Resource 2. The results show significantly higher patient satisfaction score (the sum of nine item scores corresponding to Q1, Q2, Q3, Q5, Q6, Q7, Q8, Q9, and Q12) in the 2/W-TPTD group at 2 weeks after the start of treatment (p < 0.05) but no difference in the sums of two item scores corresponding to Q10 and Q11 at any time point. Scores corresponding to Q3 (Confirmation that the injection was successful) and Q9 (Pain at the injection site) were also higher in the 2/W-TPTD group at 4 and 13 weeks, and the score corresponding to Q4 (Have you failed to inject the medication?) was lower at 4 and 26 weeks. The time course of the mean values of those two scores (Q3, 9) is shown in Fig. 1a and b.
Time course of the rate of patient satisfaction with injection device utility corresponding to a Q3 and b Q9. Each point and bar indicate the mean score ± standard deviation. Asterisk indicates p < 0.05 between the groups. 1/D-TPTD once-daily dose of teriparatide, 2/W-TPTD twice-weekly dose of teriparatide
Clinical fracture occurred in 4 patients in the 2/W-TPTD group (1 vertebral fracture and 3 non-vertebral fractures), but not the 1/D-TPTD group. However, the difference between the groups was not statistically significant (p = 0.061). QOL at 26 weeks was significantly improved from the baseline value in both groups (Table 3). The heights of patients significantly decreased at 26 weeks in the both groups, whereas body weight was significantly decreased in the 2/W-TPTD group (p = 0.001). AEs occurred in 10 patients (5.6%) in the 1/D-TPTD group and 13 patients (7.2%) in the 2/W-TPTD group, none of which were serious (Table 4).
Discussion
This is a preliminary report on our ongoing comparative crossover study to evaluate patient satisfaction with two types of teriparatide self-injection systems, the 1/D-TPTD and 2/W-TPTD. The results of evaluation conducted at 26 weeks after the start of treatment, that is, just before the crossover, demonstrated that the degree of overall patient satisfaction for both injection systems did not differ significantly between the two dosing regimens. This is the first report on the patient satisfaction with these two systems. A recent study by Gold et al. also reported similar findings with respect to patient satisfaction with abaloparatide [18]. Satisfaction with treatment also did not differ significantly between the two injection systems.
Patient satisfaction with the utility of the self-injection system was evaluated using a 12-item questionnaire. The scores in 9 out of 12 items were significantly higher in the 2/W-TPTD group than those in 1/D-TPTD group in the early phase of treatment. Although the trend persisted, the number of items with significant difference gradually decreased as treatment continued and the difference almost disappeared at 26 weeks except for the Q4 (Have you failed to inject the medication?). Those differences may have resulted from the differences between the two injection systems, in particular, in the volume and pH of the injection solution, time and effort of changing the needle, and injection frequency. At the beginning, the high frequency of injection and preparation may have had a greater impact on patients in the 1/D-TPTD group, resulting in a lower satisfaction score, but as the study progressed the patients gradually got used to the routine and scores in both groups appeared to reach similar levels. As for pain at injection, the difference in scores may have been due to a difference in solution pH, 4.4–5.3 for the 2/W-TPTD group and 3.8–4.5 for the 1/D-TPTD group.
Regarding the items for which there were significant differences in patient satisfaction with utility, it is possible that giving patients an adequate explanation of the characteristics of each injection device before administration may lead to appropriate drug selection.
The persistence rate at 26 weeks was 79.4% in the 1/D-TPTD group and 72.6% in the 2/W-TPTD group and was slightly higher than the rates in previous reports evaluating persistence of TPTDs that used real-world data from the Japanese population [12, 19]. The seemingly higher rates may be due to follow-up by the investigators in which patients were asked about how well they were coping with self-injection and explaining to patients the importance of medication adherence, which may have motivated patients to continue self-injection. If this is the case, then the same strategy should be used in real-world clinical practice. The recent report showing high adherence to abaloparatide with high satisfaction from a real-world experience perspective [18] is encouraging, with the authors noting that 55% of patients discussed treatment options in detail with their healthcare team and 27% frequently asked their healthcare team questions regarding treatment choices [18]. Similar results indicating the importance of patient education for adherence to and persistence with teriparatide therapy have been also reported [20, 21].
New clinical fracture occurred in 4 patients (2.2%) in the 2/W-TPTD group. However, these fractures were detected within 2 months after the start of the study and possibly therefore unrelated to the treatment. No statistically significant difference between the groups was observed in this regard.
Utility (EQ-5D) score and pain VAS score analysis indicates significant improvement at 26 weeks, similar to previous reports [22, 23].
Limitation
The data presented here are from a randomized, allocation-controlled study and may differ from the data obtained in actual clinical situations. However, it is interesting to note that there are some differences between the two dosing regimens (two TPTD self-injection devices). The present results were based on information obtained in the first quarter of the 2-year study period. It is hoped that the post-crossover results after the first quartile study will make the differences between the two dosing regimens with TPTD self-injection devices more compelling.
Conclusion
The present study demonstrated that overall satisfaction and effectiveness of treatment were similar between the 1/D-TPTD and 2/W-TPTD groups. The finding of a between-group difference in satisfaction with the utility of the self-injection device was useful information for the treatment of osteoporosis in real-world clinical practice. Both medication regimens were well tolerated without serious adverse events.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376. https://doi.org/10.1016/S0140-6736(18)32112-3
Reid IR, Billington EO (2022) Drug therapy for osteoporosis in older adults. Lancet 399:1080–1092. https://doi.org/10.1016/S0140-6736(21)02646-5
McClung MR, Rothman MS, Lewiecki EM, Hanley DA, Harris ST, Miller PD, Kendler DL (2022) The role of osteoanabolic agents in the management of patients with osteoporosis. Postgrad Med 134:541–551. https://doi.org/10.1080/00325481.2022.2069582
Cosman F (2020) Anabolic therapy and optimal treatment sequences for patients with osteoporosis at high risk for fracture. Endocr Pract 26:777–786. https://doi.org/10.4158/EP-2019-0596
Rabenda V, Reginster JY (2010) Overcoming problems with adherence to osteoporosis medication. Expert Rev Pharmacoecon Outcomes Res 10:677–689. https://doi.org/10.1586/erp.10.76
Alahmari MM, AlHilali AI, Thabet TA, Alshahrani MA, Mobasher WA, Al Mubarak DA, Alshamrani AM, Gohman RS, Alqarni SA, Alqahtani MM (2023) Impact of medication adherence on bone mineral density and fracture risk in patients with osteoporosis: a systematic review. Cureus. https://doi.org/10.7759/cureus.42115
Chen Q, Guo M, Ma X, Pu Y, Long Y, Xu Y (2019) Adherence to teriparatide treatment and risk of fracture: a systematic review and meta-analysis. Horm Metab Res 51:785–791. https://doi.org/10.1055/a-1062-9447
Park JH, Park EK, Koo DW, Lee S, Lee SH, Kim GT, Lee SG (2017) Compliance and persistence with oral bisphosphonates for the treatment of osteoporosis in female patients with rheumatoid arthritis. BMC Musculoskelet Disord 18:152. https://doi.org/10.1186/s12891-017-1514-4
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441. https://doi.org/10.1056/NEJM200105103441904
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97:3097–3106. https://doi.org/10.1210/jc.2011-3479
Sugimoto T, Shiraki M, Fukunaga M, Kishimoto H, Hagino H, Sone T, Nakano T, Ito M, Yoshikawa H, Minamida T, Tsuruya Y, Nakamura T (2019) Study of twice-weekly injections of teriparatide by comparing efficacy with once-weekly injections in osteoporosis patients: the TWICE study. Osteoporos Int 30:2321–2331. https://doi.org/10.1007/s00198-019-05111-6
Fujita R, Endo T, Takahata M, Haraya K, Suzuki H, Oda I, Kanayama M, Asano T, Shigenobu K, Iwata A, Yamada K, Takeuchi H, Ohura H, Yoneoka D, Iwasaki N (2022) Real-world persistence of twice-weekly teriparatide and factors associated with the discontinuation in patients with osteoporosis. J Bone Miner Metab 40:782–789. https://doi.org/10.1007/s00774-022-01347-1
Kumagai Y, Ose A, Tanaka K, Sugimoto T (2020) Safety profiles, pharmacokinetics, and changes in bone turnover markers after twice-weekly subcutaneous administration of teriparatide in healthy Japanese postmenopausal women: a single-blind randomized study. Clin Pharmacol Drug Dev 9:87–96. https://doi.org/10.1002/cpdd.687
Kendler DL, McClung MR, Freemantle N, Lillestol M, Moffett AH, Borenstein J, Satram-Hoang S, Yang YC, Kaur P, Macarios D, Siddhanti S, Investigators DAPS (2011) Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int 22:1725–1735. https://doi.org/10.1007/s00198-010-1378-z
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL, Investigators DAPS (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326. https://doi.org/10.1007/s00198-011-1780-1
Soen S, Fukunaga M, Sugimoto ST, Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, Ohta H, Yoneda T, Tomomitsu T, Japanese Society for Bone and Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257. https://doi.org/10.1007/s00774-013-0447-8
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Gold DT, Weiss R, Beckett T, Deal C, Epstein RS, James AL, Kernaghan JM, Mohseni M, Spiegel M, Vokes T, Roberts J, Bailey T, Wang Y, Williams SA (2021) Abaloparatide real world patient experience study. JBMR Plus. https://doi.org/10.1002/jbm4.10457
Usui T, Funagoshi M, Seto K, Ide K, Tanaka S, Kawakami K (2018) Persistence of and switches from teriparatide treatment among women and men with osteoporosis in the real world: a claims database analysis. Arch Osteoporos 13:54. https://doi.org/10.1007/s11657-018-0466-0
van Maren MA, Wyers CE, Driessen JHM, Visser JV, De Vries F, van de Wijdeven K, Gevers S, Lems WF, Emmelot-Vonk MH, van den Bergh JP (2019) Two-year persistence with teriparatide improved significantly after introduction of an educational and motivational support program. Osteoporosis Int 30:1837–1844. https://doi.org/10.1007/s00198-019-05052-0
Sato M, Tsujimoto M, Kajimoto K, Uetake H, Shimoda H, Fujiwara S (2018) Effect of a patient-support program on once-daily teriparatide adherence and persistence in the Japan Fracture Observational Study (JFOS). Arch Osteoporos 13:74. https://doi.org/10.1007/s11657-018-0487-8
Rajzbaum G, Grados F, Evans D, Liu-Leage S, Petto H, Augendre-Ferrante B (2014) Treatment persistence and changes in fracture risk, back pain, and quality of life amongst patients treated with teriparatide in routine clinical care in France: results from the European Forsteo Observational Study. Joint Bone Spine 81:69–75. https://doi.org/10.1016/j.jbspin.2013.05.001
Soen S, Fujiwara S, Takayanagi R, Kajimoto K, Tsujimoto M, Kimura S, Sato M, Krege JH, Enomoto H (2017) Real-world effectiveness of daily teriparatide in Japanese patients with osteoporosis at high risk for fracture: final results from the 24-month Japan Fracture Observational Study (JFOS). Curr Med Res Opin 33:2049–2056. https://doi.org/10.1080/03007995.2017.1354826
Acknowledgements
This study was funded by the Public Health Research Foundation and Asahi Kasei Pharma Corp. The authors express thanks to Dr. Itsuo Gorai (chairman), Dr. Masaaki Inaba, and Dr. Kentaro Sakamaki of the independent data monitoring committee, and Dr. Eiji Itoi of the audit committee, for the JOINT-06. The authors would like to express their sincere gratitude to those who participated as clinical investigators in JOINT-06 and Mr. Masafumi Mori and Ms. Yuko Iwata at the Public Health Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Statistical analyses were performed by YU. The questionnaire was developed by JT, SI, JI, and NO. SS wrote the first draft. All authors discussed the results, and read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
S Soen has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mochida Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd. Shiro Tanaka has received lecture fees from the Research Institute of Healthcare Data Science. He has received consultation fees and outsourcing fees from Eli Lilly and Company, Welby, Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., Satt, and the Public Health Research Foundation. He has received research grants from the Japan Agency for Medical Research and Development, the Japanese Ministry of Health, Labour and Welfare, the Japanese Ministry of Education, Science and Technology, and Novo Nordisk. He engaged in a research project of the Japan Agency for Medical Research and Development. Y Takeuchi has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mochida Pharma Co., Ltd., and Teijin Pharma Ltd., Taisho Pharmaceutical Holdings Co. Ltd., Towa Pharmaceutical Co. Ltd. J Takada has received consulting fees and speaking fees from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd. S Ikeda has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Pfizer Inc., Hisamitsu Pharmaceutical Co., Inc., Mochida Pharma Co., Ltd., Teijin Pharma Ltd., Towa Pharmaceutical Co., Ltd., and UCB Japan Co., Ltd. J Iwamoto has received speaking fees from Asahi Kasei Pharma Corp., Eisai Co., Ltd., Taisho Pharmaceutical Holdings Co., Ltd., Teijin Pharma Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kaken Pharmaceutical Co., Ltd., Amgen Inc., Astellas Pharma Inc., Towa Pharmaceutical Co., Ltd. N Okimoto has received payments for lectures, including speakers' bureau fees from Asahi Kasei Pharma Corp., Amgen Inc., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan, and Teijin Pharma Ltd. Sakae Tanaka has received lecture fees from Teijin Pharma Ltd., Asahi Kasei Pharma Corp., Amgen Inc., and Daiichi Sankyo Co., Ltd., and research grants from Teijin Pharma Ltd., Asahi Kasei Pharma Corp., Daiichi Sankyo Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Y Uemura and N Endo have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Soen, S., Uemura, Y., Tanaka, S. et al. A crossover comparison of patient satisfaction with two teriparatide regimens: primary results of the Japanese Osteoporosis Intervention Trial 06 (JOINT-06). J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01521-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01521-7