Abstract
Introduction
Aromatase inhibitors are used post-surgical intervention in postmenopausal patients with breast cancer. However, these drugs accelerate decline in bone mineral density (BMD), which is countered by use of denosumab, and the efficacy of the drug can be assessed by bone turnover markers. We investigated the effects of denosumab administration for 2 years on BMD and urinary N-telopeptide of type I collagen (u-NTX) levels in breast cancer patients treated with aromatase inhibitors.
Materials and methods
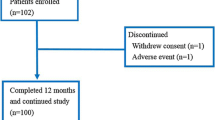
This was a single-center retrospective study. Postoperative hormone receptor-positive breast cancer patients with low T-scores biannually received denosumab from the time of initiation of aromatase inhibitor therapy for 2 years. BMD was measured every 6 months, and u-NTX levels were assessed after 1 month and thereby every 3 months.
Results
The median patient age of the 55 patients included in this study was 69 (range: 51–90) years. BMD gradually increased in the lumbar spine and femoral neck and u-NTX levels were lowest at 3 months post-initiation of therapy. Patients were divided into two groups based on the change ratio of u-NTX 3 months post-denosumab administration. Of these, the group with higher change ratio showed a higher degree of BMD restoration in the lumbar spine and femoral neck 6 months post-denosumab treatment.
Conclusion
Denosumab increased BMD in patients treated with aromatase inhibitors. The u-NTX level decreased soon after start of denosumab treatment, and its change ratio is predictive of improvement in BMD.



Similar content being viewed by others
References
Ciuba A, Wnuk K, Nitsch-Osuch A, Kulpa M (2022) Health care accessibility and breast cancer mortality in Europe (in Eng). Int J Environ Res Public Health. https://doi.org/10.3390/ijerph192013605
(2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials (in Eng). Lancet 386:1341–1352. https://doi.org/10.1016/s0140-6736(15)61074-1
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Láng I, Wardley A, Rabaglio M, Price KN, Gelber RD, Coates AS, Thürlimann B (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8·1 years median follow-up (in Eng). Lancet Oncol 12:1101–1108. https://doi.org/10.1016/s1470-2045(11)70270-4
Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE (2006) Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230) (in Eng). J Bone Miner Res 21:1215–1223. https://doi.org/10.1359/jbmr.060508
Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M, Fitterman N, Harris RP, Humphrey LL, Kansagara D, McLean RM, Mir TP, Schünemann HJ (2017) Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians (in Eng). Ann Intern Med 166:818–839. https://doi.org/10.7326/m15-1361
Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP et al (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension (in Eng). J Bone Miner Res 27:694–701. https://doi.org/10.1002/jbmr.1479
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R et al (2015) Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial (in Eng). Lancet 386:433–443. https://doi.org/10.1016/s0140-6736(15)60995-3
Ouchi Y, Nakatsukasa K, Sakaguchi K, Morita M, Koyama H, Matsuda T, Kato M, Konishi E, Ono H, Taguchi T (2021) The effect of denosumab in breast cancer patients receiving adjuvant aromatase inhibitors: 36-month results (in Eng). J Bone Miner Metab 39:224–229. https://doi.org/10.1007/s00774-020-01138-6
Kim SW, Park DJ, Park KS, Kim SY, Cho BY, Lee HK, Shin CS (2005) Early changes in biochemical markers of bone turnover predict bone mineral density response to antiresorptive therapy in Korean postmenopausal women with osteoporosis (in Eng). Endocr J 52:667–674. https://doi.org/10.1507/endocrj.52.667
Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E, Uebelhart D, Wang A, Weryha G, Cummings SR (2011) Effects of denosumab on bone turnover markers in postmenopausal osteoporosis (in Eng). J Bone Miner Res 26:530–537. https://doi.org/10.1002/jbmr.251
Shizuku M, Shibata M, Okumura M, Takeuchi D, Kikumori T, Mizuno Y (2020) Utility of urinary type I collagen cross-linked N-telopeptide as a prognostic indicator in breast cancer patients with bone metastases (in Eng). Breast Cancer 27:1065–1071. https://doi.org/10.1007/s12282-020-01109-9
Regan MM, Fleming GF, Walley B, Francis PA, Pagani O (2019) Adjuvant systemic treatment of premenopausal women with hormone receptor-positive early breast cancer: lights and shadows (in Eng). J Clin Oncol 37:862–866. https://doi.org/10.1200/jco.18.02433
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230 (in Eng). J Clin Oncol 26:1051–1057. https://doi.org/10.1200/jco.2007.11.0726
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results (in Eng). Ann Oncol 24:398–405. https://doi.org/10.1093/annonc/mds277
Gnant M, Pfeiler G, Steger GG, Egle D, Greil R, Fitzal F, Wette V, Balic M, Haslbauer F, Melbinger-Zeinitzer E, Bjelic-Radisic V, Jakesz R, Marth C, Sevelda P, Mlineritsch B, Exner R, Fesl C, Frantal S, Singer CF (2019) Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial (in Eng). Lancet Oncol 20:339–351. https://doi.org/10.1016/s1470-2045(18)30862-3
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass (in Eng). J Clin Endocrinol Metab 96:972–980. https://doi.org/10.1210/jc.2010-1502
Nakatsukasa K, Koyama H, Ouchi Y, Sakaguchi K, Fujita Y, Matsuda T, Kato M, Konishi E, Taguchi T (2018) Effect of denosumab administration on low bone mineral density (T-score − 1.0 to − 2.5) in postmenopausal Japanese women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer (in Eng). J Bone Miner Metab 36:716–722. https://doi.org/10.1007/s00774-017-0884-x
Generali D, Berruti A, Tampellini M, Dovio A, Tedoldi S, Bonardi S, Tucci M, Allevi G, Aguggini S, Milani M, Bottini A, Dogliotti L, Angeli A (2007) The circadian rhythm of biochemical markers of bone resorption is normally synchronized in breast cancer patients with bone lytic metastases independently of tumor load. Bone 40:182–188. https://doi.org/10.1016/j.bone.2006.06.023
Coleman R, Brown J, Terpos E, Lipton A, Smith MR, Cook R, Major P (2008) Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions (in Eng). Cancer Treat Rev 34:629–639. https://doi.org/10.1016/j.ctrv.2008.05.001
McCaig FM, Renshaw L, Williams L, Young O, Murray J, Macaskill EJ, McHugh M, Hannon R, Dixon JM (2010) A study of the effects of the aromatase inhibitors anastrozole and letrozole on bone metabolism in postmenopausal women with estrogen receptor-positive breast cancer (in Eng). Breast Cancer Res Treat 119:643–651. https://doi.org/10.1007/s10549-009-0646-0
Lipton A, Smith MR, Fizazi K, Stopeck AT, Henry D, Brown JE, Shore ND, Saad F, Spencer A, Zhu L, Warner DJ (2016) Changes in bone turnover marker levels and clinical outcomes in patients with advanced cancer and bone metastases treated with bone antiresorptive agents. Clin Cancer Res 22:5713–5721. https://doi.org/10.1158/1078-0432.ccr-15-3086
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension (in Eng). J Bone Miner Res 33:190–198. https://doi.org/10.1002/jbmr.3337
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC (2017) Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS (in Eng). Bone 105:11–17. https://doi.org/10.1016/j.bone.2017.08.003
Burckhardt P, Faouzi M, Buclin T, Lamy O (2021) Fractures after denosumab discontinuation: a retrospective study of 797 cases (in Eng). J Bone Miner Res 36:1717–1728. https://doi.org/10.1002/jbmr.4335
Acknowledgements
We thank the patients and their families for their participation in this study.
Funding
The authors declare that they did not receive any funding for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: YM; data curation: MO, SK, HN, CT, YM; methodology: MS, MO, YM; formal analysis and investigation: MS, MO, YM; writing—original draft preparation: MS; writing—review and editing: MO, SK, HN, CT, YM; supervision: YM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have stated that they have no conflicts of interest to declare.
Ethical approval
This study was approved by the Institutional Review Board Committee of the Yokkaichi Municipal Hospital (No. 2017-18). All procedures involving human participants were performed in accordance with the ethical standards of the institutional committee and the Declaration of Helsinki. As this was a retrospective study, formal consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
774_2023_1429_MOESM1_ESM.pptx
Supplementary Fig. 1 Correlation between clinical factors and bone mineral density (BMD) change ratios after six months. There was no significant correlation between BMD change ratios and age (a), years since menopause (b), or body mass index (c) (Spearman's rank correlation test.). (PPTX 94 KB)
774_2023_1429_MOESM2_ESM.pptx
Supplementary Fig. 2 Association between clinical factors and bone mineral density (BMD) change ratios after six months. There was no significant association between BMD change ratios and history of fracture (a) or type of aromatase inhibitors (b) (N.S., not significant, Mann–Whitney U test.). (PPTX 80 KB)
About this article
Cite this article
Shibata, M., Okumura, M., Kawano, S. et al. Denosumab effect on bone mineral density and urinary-NTX in breast cancer patients receiving aromatase inhibitors. J Bone Miner Metab 41, 567–574 (2023). https://doi.org/10.1007/s00774-023-01429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01429-8