Abstract
Introduction
A disintegrin and metalloproteinase 17 (Adam17), also known as TNFα-converting enzyme (Tace), is a membrane-anchored protein involved in shedding of TNF, IL-6 receptor, ligands of epidermal growth factor receptor (EGFR), and Notch receptor. This study aimed to examine the role of Adam17 in adult articular cartilage and osteoarthritis (OA) pathophysiology.
Materials and methods
Adam17 expression was examined in mouse knee joints during OA development. We analyzed OA development in tamoxifen-inducible chondrocyte-specific Adam17 knockout mice of a resection of the medial meniscus and medial collateral ligament (medial) model, destabilization of the medial meniscus (DMM) model, and aging model. We analyzed downstream pathways by in vitro experiments, and further performed intra-articular administration of an Adam17 inhibitor TAPI-0 for surgically induced mouse OA.
Results
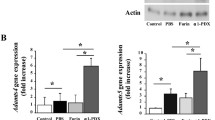
Adam17 expression in mouse articular cartilage was increased by OA progression. In all models, Adam17 knockout mice showed ameliorated progression of articular cartilage degradation. Adam17 knockout decreased matrix metallopeptidase 13 (Mmp13) expression in both in vivo and in vitro experiments, whereas Adam17 activation by phorbol-12-myristate-13-acetate (PMA) increased Mmp13 and decreased aggrecan in mouse primary chondrocytes. Adam17 activation enhanced release of soluble TNF and transforming growth factor alpha, a representative EGF ligand, from mouse primary chondrocytes, while it did not change release of soluble IL-6 receptor or nuclear translocation of Notch1 intercellular domain. Intra-articular administration of the Adam17 inhibitor ameliorated OA progression.
Conclusions
This study demonstrates regulation of OA development by Adam17, involvement of EGFR and TNF pathways, and the possibility of Adam17 as a therapeutic target for OA.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S (2016) Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 59:134–138
Chang SH, Mori D, Kobayashi H, Mori Y, Nakamoto H, Okada K, Taniguchi Y, Sugita S, Yano F, Chung UI, Kim-Kaneyama JR, Yanagita M, Economides A, Canalis E, Chen D, Tanaka S, Saito T (2019) Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-kappaB pathway. Nat Commun 10:1442
Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung UI, Kawaguchi H (2010) Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med 16:678–686
Kobayashi H, Chang SH, Mori D, Itoh S, Hirata M, Hosaka Y, Taniguchi Y, Okada K, Mori Y, Yano F, Chung UI, Akiyama H, Kawaguchi H, Tanaka S, Saito T (2016) Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat Commun 7:13336
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H (2013) Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci USA 110:1875–1880
Sugita S, Hosaka Y, Okada K, Mori D, Yano F et al (2015) Transcription factor Hes1 modulates osteoarthritis development in cooperation with calcium/calmodulin-dependent protein kinase 2. Proc Natl Acad Sci USA 112:3080–3085
Qin L, Beier F (2019) EGFR signaling: friend or foe for cartilage? JBMR Plus 3:e10177
Jia H, Ma X, Tong W, Doyran B, Sun Z, Wang L, Zhang X, Zhou Y, Badar F, Chandra A, Lu XL, Xia Y, Han L, Enomoto-Iwamoto M, Qin L (2016) EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc Natl Acad Sci USA 113:14360–14365
Sun H, Wu Y, Pan Z, Yu D, Chen P, Zhang X, Wu H, Zhang X, An C, Chen Y, Qin T, Lei X, Yuan C, Zhang S, Zou W, Ouyang H (2018) Gefitinib for epidermal growth factor receptor activated osteoarthritis subpopulation treatment. EBioMedicine 32:223–233
Lisi S, D’Amore M, Sisto M (2014) ADAM17 at the interface between inflammation and autoimmunity. Immunol Lett 162:159–169
Ohta S, Harigai M, Tanaka M, Kawaguchi Y, Sugiura T, Takagi K, Fukasawa C, Hara M, Kamatani N (2001) Tumor necrosis factor-alpha (TNF-alpha) converting enzyme contributes to production of TNF-alpha in synovial tissues from patients with rheumatoid arthritis. J Rheumatol 28:1756–1763
Murumkar PR, Ghuge RB, Chauhan M, Barot RR, Sorathiya S, Choudhary KM, Joshi KD, Yadav MR (2020) Recent developments and strategies for the discovery of TACE inhibitors. Expert Opin Drug Discov 15:779–801
Moss ML, Minond D (2017) Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm 2017:9673537
Nakamoto H, Katanosaka Y, Chijimatsu R, Mori D, Xuan F, Yano F, Omata Y, Maenohara Y, Murahashi Y, Kawaguchi K, Yamagami R, Inui H, Taketomi S, Taniguchi Y, Kanagawa M, Naruse K, Tanaka S, Saito T (2021) Involvement of transient receptor potential Vanilloid channel 2 in the induction of lubricin and suppression of ectopic endochondral ossification in mouse articular cartilage. Arthritis Rheumatol 73:1441–1450
Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP (2007) Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol 179:2686–2689
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D (2009) Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res 24:12–21
Glasson SS, Blanchet TJ, Morris EA (2007) The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15:1061–1069
Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H (2005) Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage 13:632–641
Glasson SS, Chambers MG, Van Den Berg WB, Little CB (2010) The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18:S17-23
Gosset M, Berenbaum F, Thirion S, Jacques C (2008) Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3:1253–1260
Shao MX, Ueki IF, Nadel JA (2003) Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 100:11618–11623
Stanton H, Golub SB, Rogerson FM, Last K, Little CB, Fosang AJ (2011) Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat Protoc 6:388–404
Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M et al (1994) Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 370:218–220
Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM (1995) A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol 155:5198–5205
Doedens JR, Black RA (2000) Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J Biol Chem 275:14598–14607
Zhang X, Zhu J, Liu F, Li Y, Chandra A, Levin LS, Beier F, Enomoto-Iwamoto M, Qin L (2014) Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res 2:14015
Appleton CT, Usmani SE, Bernier SM, Aigner T, Beier F (2007) Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum 56:3693–3705
Usmani SE, Ulici V, Pest MA, Hill TL, Welch ID, Beier F (2016) Context-specific protection of TGFalpha null mice from osteoarthritis. Sci Rep 6:30434
Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP et al (2018) Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet 50:549–558
Saito K, Horiuchi K, Kimura T, Mizuno S, Yoda M, Morioka H, Akiyama H, Threadgill D, Okada Y, Toyama Y, Sato K (2013) Conditional inactivation of TNFalpha-converting enzyme in chondrocytes results in an elongated growth plate and shorter long bones. PLoS ONE 8:e54853
Hall KC, Hill D, Otero M, Plumb DA, Froemel D, Dragomir CL, Maretzky T, Boskey A, Crawford HC, Selleri L, Goldring MB, Blobel CP (2013) ADAM17 controls endochondral ossification by regulating terminal differentiation of chondrocytes. Mol Cell Biol 33:3077–3090
Usmani SE, Pest MA, Kim G, Ohora SN, Qin L, Beier F (2012) Transforming growth factor alpha controls the transition from hypertrophic cartilage to bone during endochondral bone growth. Bone 51:131–141
Bozkulak EC, Weinmaster G (2009) Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol 29:5679–5695
Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israël A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5:207–216
Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T (1994) Notch1 is essential for postimplantation development in mice. Genes Dev 8:707–719
Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T (1995) Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 121:3291–3301
Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM (1998) TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 160:943–952
Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lübke T, Lena Illert A, von Figura K, Saftig P (2002) The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet 11:2615–2624
Acknowledgements
We thank Dr. Di Chen for generously providing Col2a1-CreERT2 mice. We thank J. Sugita, K. Kaneko, and R. Homma for technical assistance. We also thank Mitchell Arico from Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers 20H03799, 19H05654, 19H05565, 19K09641, 18KK0254, 17H04310, and 26462285.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript. TK, KH, and TS conceived and designed the study. TK, RC, DM, and KN performed the experiment. TK, RC, FY, HI, TM, ST, and TS analyzed and interpreted the data. TK and TS wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kaneko, T., Horiuchi, K., Chijimatsu, R. et al. Regulation of osteoarthritis development by ADAM17/Tace in articular cartilage. J Bone Miner Metab 40, 196–207 (2022). https://doi.org/10.1007/s00774-021-01278-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01278-3