Abstract
Introduction
Although aromatase inhibitors (AIs) are typical drugs for cancer treatment-induced bone loss, their effects on the bone microstructure remain unclear. In this study, we evaluated changes in the bone mineral density (BMD) and bone microstructure associated with AI treatment using high-resolution peripheral quantitative computed tomography (HR-pQCT) in patients with early breast cancer.
Materials and methods
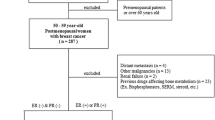
This prospective, single-arm, observational study included non-osteoporotic, postmenopausal women with hormone receptor-positive breast cancer. Patients underwent dual-energy X-ray absorptiometry (DXA), HR-pQCT, and tartrate-resistant acid phosphatase-5b (TRACP-5b) or procollagen type-I N-terminal propeptide measurements at baseline and 6 and 12 months after AI therapy. The primary endpoint was changes in the total volumetric BMD (Tt.vBMD), trabecular vBMD (Tb.vBMD), and cortical vBMD (Ct.vBMD) longitudinally at the distal radius and tibia.
Results
Twenty women were included (median age 57.5 years; range 55–72 years). At 12 months, HR-pQCT indicated a significant decrease in the Tt.vBMD (median distal radius − 5.3%, p < 0.01; distal tibia − 3.2%, p < 0.01), Tb.vBMD (− 3.2%, p < 0.01; − 1.0%, p < 0.05, respectively), and Ct.vBMD (− 3.2%, p < 0.01; − 2.7%, p < 0.01, respectively). Estimated bone strength was also significantly decreased. The DXA BMD value in the total hip (p < 0.01) and femoral neck (p = 0.03), but not in the lumbar spine, was significantly decreased. The TRACP-5b levels was significantly negatively associated with changes in the Tt.vBMD in both the distal radius and tibia (r = − 0.53, r = − 0.47, respectively)
Conclusion
Postmenopausal women who received AIs for early breast cancer experienced significant trabecular and cortical bone deterioration and a decrease in estimated bone strength within only 1 year.


Similar content being viewed by others
Abbreviations
- BMD:
-
Bone mineral density
- BV/TV:
-
Trabecular bone volume fraction
- CI:
-
Confidence interval
- Ct.Ar:
-
Cortical area
- Ct.Pm:
-
Cortical perimeter
- Ct.Po.Dm:
-
Cortical pore diameter
- Ct.Po:
-
Cortical porosity
- Ct.Th:
-
Cortical thickness
- Ct.vBMD:
-
Cortical volumetric bone mineral density
- DXA:
-
Dual-energy X-ray absorptiometry
- HR( +) EBC:
-
Hormone receptor-positive early breast cancer
- HR-pQCT:
-
High-resolution peripheral quantitative computed tomography
- OR:
-
Odds ratio
- P1NP:
-
Procollagen type-I N-terminal propeptide
- Tb.Inn.BMD:
-
Inner trabecular bone mineral density
- Tb.Meta.BMD:
-
Meta trabecular bone mineral density
- Tb.N:
-
Trabecular number
- Tb.Sp:
-
Trabecular separation
- Tb.Th:
-
Trabecular thickness
- Tb.vBMD:
-
Trabecular volumetric bone mineral density
- TRACP-5b:
-
Tartrate-resistant acid phosphatase-5b
- Tt.vBMD:
-
Total volumetric bone mineral density
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ (2019) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37:423–438
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Chien AJ, Goss PE (2006) Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol 24:5305–5312
Goldvaser H, Barnes TA, Šeruga B, Cescon DW, Ocaña A, Ribnikar D, Amir E (2018) Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst 110:31–39
Kuba S, Maeda S, Matsumoto M, Yamanouchi K, Yano H, Morita M, Sakimura C, Hatachi T, Tokai Y, Takatsuki M, Fujioka H, Hayashida N, Nagayasu T, Eguchi S (2018) Adherence to adjuvant endocrine therapy in women with breast cancer: a prospective observational study in Japanese women. Clin Breast Cancer 18:150–156
Eastell R, Adams J, Clack G, Howell A, Cuzick J, Mackey J, Beckmann MW, Coleman RE (2011) Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol 22:857–862
Zaman K, Thürlimann B, Huober J, Schönenberger A, Pagani O, Lüthi J, Simcock M, Giobbie-Hurder A, Berthod G, Genton C, Brauchli P, Aebi S, Swiss Group for Clinical Cancer Research (SAKK) (2012) Bone mineral density in breast cancer patients treated with adjuvant letrozole, tamoxifen, or sequences of letrozole and tamoxifen in the BIG 1–98 study (SAKK 21/07). Ann Oncol 23:1474–1481
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R et al (2015) Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386:433–443
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2000) Osteoporosis prevention, diagnosis, and therapy. JAMA 17:1–36
Cheung AM, Tile L, Cardew S, Pruthi S, Robbins J, Tomlinson G, Kapral MK, Khosla S, Majumdar S, Erlandson M, Scher J, Hu H, Demaras A, Lickley L, Bordeleau L, Elser C, Ingle J, Richardson H, Goss PE (2012) Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol 3:275–284
Ramchand SK, Seeman E, Wang XF, Ghasem-Zadeh A, Francis PA, Ponnusamy EJ, Bardin MS, Bui M, Zebaze R, Zajac JD, Grossmann M (2017) Premenopausal women with early breast cancer treated with estradiol suppression have severely deteriorated bone microstructure. Bone 103:131–135
Chiba K, Okazaki N, Kurogi A, Isobe Y, Yonekura A, Tomita M, Osaki M (2018) Precision of second-generation high-resolution peripheral quantitative computed tomography: intra- and intertester reproducibilities and factors involved in the reproducibility of cortical porosity. J Clin Densitom 21:295–302
Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S (2012) Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone 50:111–118
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Yokota K, Chiba K, Okazaki N, Kondo C, Doi M, Yamada S, Era M, Nishino Y, Yonekura A, Tomita M, Osaki M (2020) Deterioration of bone microstructure by aging and menopause in Japanese healthy women: analysis by HR-pQCT. J Bone Miner Metab 38:826–838
Samelson EJ, Broe KE, Xu H, Yang L, Boyd S (2019) Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7:34–43
Nenonen A, Cheng S, Ivaska KK, Alatalo SL, Lehtimäki T, Schmidt-Gayk H, Uusi-Rasi K, Heinonen A, Kannus P, Sievänen H, Vuori I, Väänänen HK, HalleeN JM (2015) Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J Bone Miner Res 20:1804–1812
Ouchi Y, Nakatsukasa K, Sakaguchi K, Morita M, Koyama H, Matsuda T, Kato M, Konishi E, Ono H, Tetsuya Taguchi T (2021) The effect of denosumab in breast cancer patients receiving adjuvant aromatase inhibitors: 36-month results. J Bone Miner Meta 39:224–229
Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R (2010) Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol 28:967–975
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24:398–405
Gonzalez-Rodriguez E, Aubry-Rozier B, Stoll D, Zaman K, Lamy O (2020) Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res Treat 179:153–159
Acknowledgements
The authors thank Ms. Haraguchi for creating and managing the database. The authors also thank Editage Group (https://www.editage.jp/) for editing a draft of this manuscript. This research was supported by a “Japan Osteoporosis Foundation Grant for Bone Research.” The sponsor had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
SK, WK, CK, Megumi Matsumoto, and KY were responsible for conceiving and designing the trial and planning data analysis. SK, Megumi Matsumoto, KY, AF, XM, Michi Morita, RO, and HY participated in data collection and were in charge of patient recruitment and treatment. KW and KC were responsible for HR-pQCT analysis. KK, MO, TN, and SE were responsible for planning the data analysis and analyzing the data resulting from the trial. All authors have contributed to and approved the final version of this manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Ethical approval
This study was approved by the Nagasaki University Hospital Clinical Research Ethical Committee (17041711). The protocol was designed and conducted in accordance with the Declaration of Helsinki (1964) and its later amendments.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kuba, S., Watanabe, K., Chiba, K. et al. Adjuvant endocrine therapy effects on bone mineral density and microstructure in women with breast cancer. J Bone Miner Metab 39, 1031–1040 (2021). https://doi.org/10.1007/s00774-021-01239-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01239-w