Abstract
Background
The complex endovascular repair of aortic aneurysms and dissections with fenestrated or branched stent grafts (FB-EVAR) remains challenging for interventional vascular surgery. To date, the evidence regarding treatment patterns and outcome measures consists of single center studies; however, it might be reasonable to validate results with multicenter real-world evidence.
Methods
Health insurance claims data from Germany’s third largest insurance provider, DAK-Gesundheit, were used to determine outcomes following FB-EVAR of non-ruptured thoracic aorta (TA) or thoracoabdominal including pararenal abdominal (TAA) aorta. The study included patients operated between January 2008 and April 2017.
Results
Included were 984 patients (18.1% female) who underwent FB-EVAR. Patients with treatment of the TA were younger (71.7 vs. 73.2 years, p < 0.001) and more often female (38.5% vs. 17.0%, p < 0.001) as compared to patients with treatment of TAA. In the TA group peripheral arterial disease was less frequent compared to the TAA group (67.3% vs. 80.4%, p = 0.036). Mortality was significantly (p < 0.001) higher following repair of the TAA compared to the TA at discharge (17.3% vs. 4.6%), at 30 days (26.9% vs. 8.2%) and at 90 days (34.6% vs. 10.1%). Patients with treatment of the TAA suffered more often from stroke as compared to the TA group (7.7% vs. 1.2%, p = 0.002).
Conclusion
In this large-scale German analysis of claims data, multicenter real-world evidence was different from single center studies regarding patient risk-factors and outcome measures. Validated multicenter registry studies could help to further investigate this topic in times of increasing procedures.
Zusammenfassung
Hintergrund
Die komplexe endovaskuläre Versorgung von Aneurysmen und Dissektionen der Aorta mithilfe von fenestrierten oder gebranchten Endoprothesen (FB-EVAR) ist noch immer eine Herausforderung für die endovaskuläre Gefäßchirurgie. Bisher besteht die Evidenzbasis zu diesem Thema weitestgehend aus Single-Center-Studien, die mit einer multizentrischen Versorgungsrealität verglichen werden sollte.
Methoden
Die Daten zu stationären Behandlungen der drittgrößten gesetzlichen Krankenversicherung Deutschlands, DAK-Gesundheit, wurden ausgewertet, um komplexe endovaskuläre Behandlungen der thorakalen (TA) und thorakoabdominellen inklusive der Viszeralgefäße einbeziehenden abdominellen (TAA) Aorta zu analysieren.
Ergebnisse
Zwischen Januar 2008 und April 2017 wurden insgesamt 984 Patienten (18,1 % davon weiblich) endovaskulär mithilfe von fenestrierten oder gebranchten Endoprothesen an der TA oder TAA behandelt. Patienten mit Versorgung der TA waren etwas jünger (71,7 vs. 73,2 Jahre, p < 0,001) und häufiger weiblichen Geschlechts (38,5 % vs. 17,0 %, p < 0,001) als Patienten mit Versorgung der TAA. Bei TA wurde seltener eine periphere (atherosklerotische) Gefäßerkrankung dokumentiert (67,3 % vs. 80,4 %, p = 0,036). Die Krankenhaus- (17,3 % vs. 4,6 %), 30-Tages- (26,9 % vs. 8,2 %) und 90-Tages-Sterblichkeit (34,6 % vs. 10,1 %) war signifikant höher bei Behandlung der TA im Vergleich zur TAA. Die Rate an Schlaganfällen und transienten ischämischen Attacken war höher bei Versorgung der TA (7,7 % vs. 1,2 %, p = 0,002) im Vergleich zur Versorgung der TAA.
Schlussfolgerung
In dieser großen Routinedatenanalyse zur Darstellung der multizentrischen Versorgungsrealität zeigten sich relevante Unterschiede sowohl hinsichtlich Patientenalter, Geschlecht und Sterblichkeit zwischen den analysierten Gruppen (TA vs. TAA) als auch im Vergleich mit den derzeit verfügbaren Studienergebnissen. Multizentrische validierte Registerstudien zum Abgleich von Primär- und Sekundärdatenquellen sind zu empfehlen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Aortic aneurysms and dissections that may involve supra-aortic or visceral branches and which require treatment are of central importance in interdisciplinary vascular medicine. As such, their epidemiology [29] as well as their treatment have changed fundamentally over the last few decades [27]. The statistics on procedure-specific diagnosis related groups (DRG) compiled by the German Federal Statistical Office (Statistisches Bundesamt, DeStatis) in Wiesbaden have for years been showing a rising number of annual procedures coded for thoracoabdominal pathologies (Fig. 1; [12]). Besides the strictly infrarenal or thoracic aortic aneurysms that do not involve the visceral segment or supra-aortic branches, these complex pathologies represent a particular challenge in interventional vascular surgery [2]. This entity, as well as its successful open management, was first described as early as 1955 by the vascular surgeon Stephen N. Etheredge (Oakland, California) [16]. Thoracoabdominal aneurysms can be classified into types I–V according to the Crawford classification (modified according to Safi) [11, 26]. Today, a variety of minimally invasive procedures are available for endovascular aortic repair (EVAR), (Fig. 2), whereas 15 years ago complex aneurysm repair was mostly still performed in an open procedure (open aortic repair, OAR). Technical advances in the endografts available also result in increased demands on the surgeon’s interventional experience and the infrastructure of the treating center. Against the backdrop of an ever-aging population with increasing life expectancy, this progress is the subject of controversy. This original article provides an overview of complex endovascular repair of intact aortic aneurysms and aortic dissections in German hospitals using claims data from the third largest German statutory health insurance, DAK-Gesundheit (DAK-G).
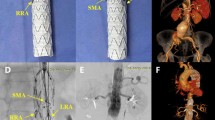
a Three-dimensional reconstruction of a branched endograft placed in the aortic arch and occlusion of the left subclavian aorta using a plug (arrow 1). b Three-dimensional reconstruction of a long four-branched thoracoabdominal endograft with overstenting and occlusion of the left subclavian artery (arrow 2 candy plug placed in the false lumen, arrow 3 celiac artery, arrow 4 superior mesenteric artery, arrow 5 left renal artery, arrow 6 right renal artery)
Methods
Study population and statistics
The database of the DAK-G, Germany’s third largest statutory health insurance (SHI), contains all outpatient and inpatient procedures performed on 6.5 million insured persons (accounting for 8% of all inhabitants in Germany). The DAK‑G database has previously been used for studies on abdominal aortic aneurysms (AAA) [7, 30], Lyme disease [23], skin cancer [1], and severe psychiatric disorders [17]. The DAK-G data can be used to create a population reference to the SHI population, showing comparable gender and age distributions (40.4% female, 29.1% ≥ 65 years).
All claims for inpatient hospital treatment according to § 301 and § 115 of the German Social Code (Sozialgesetzbuch, SGB) V submitted between January 2008 and April 2017 with the World Health Organization (WHO) International Classification of Diseases 10 (ICD-10) diagnosis of thoracic (TA, I71.1, I71.2), thoracoabdominal (TAA, I71.5, I71.6), or abdominal (AAA, I71.3, I71.4) aortic aneurysm, or with the WHO ICD-10 diagnosis of thoracic (dTA, I71.01, I71.05), thoracoabdominal (dTAA, I71.03, I71.07), or abdominal (dAA, I71.02, I71.06) aortic dissection or to which a German operation and procedure key (Operationen- und Prozedurenschlüssel, OPS) for a complex endovascular aortic repair was coded (Table 1), were included in the selection. Patients diagnosed with rupture were subsequently excluded from further analysis, thereby ensuring that only intact aneurysms or dissections were considered.
The patient selection only considers intact aneurysms or dissections
The patient selection only considers intact aneurysms or dissections based on the localization of endovascular repair, the study population was divided into thoracic procedures (TA, complex aortic arch repair) and thoracoabdominal procedures (TAA). Abdominal aortic procedures involving the visceral vessel segment were assigned to the TAA group. The German OPS code is based on the international classification of procedures in medicine (ICPM). Administrative and demographic data (age, gender), primary and secondary procedures, case-based diagnoses as well as reasons for discharge were collected for all cases identified. The first procedure submitted was deemed an index procedure. The Elixhauser comorbidity index [15, 25], which enables the uniform classification of WHO ICD-10 codes into 30 categories, was used to measure comorbidity. The linear comorbidity score according to van Walraven et al. [31] was then used to create a metric covariate from the coded comorbidities (−19 to +89).
Ethical aspects
Since the project is a retrospective analysis of anonymized statutory health insurance parameters collected in the context of routine procedures, it does not represent research on humans and does not fall under research projects requiring consultation. Therefore, in accordance with applicable case law, no ethics approval is required and patient consent was not obtained. The study group is not able to identify individual subjects on the basis of the available data.
Results
According to the DAK database, 984 patients underwent complex endovascular repair for intact aortic aneurysms between January 2008 and April 2017. In total, 52 cases (5.3%) of isolated TA involving supra-aortic vessels (aortic arch) were treated, while 932 cases (94.7%) of TAA or abdominal aorta involving visceral vessels were treated. Table 2 shows patient characteristics and risk factors. The average patient age was 71.7 years at the time of TA repair and 73.2 years at TAA repair (p < 0.001). The percentage of male patients was lower in the TA group (61.5% vs. 83.0% for TAA, p < 0.001). With the exception of a higher rate of peripheral vascular disease (80.4% vs. 67.3%, p = 0.036) in the TAA group, there were no significant differences in terms of comorbidities. At 6.46 and 6.86 points (p = 0.689), respectively, the van Walraven comorbidity index was comparable in the two groups.
Table 3 shows hospital mortality and relevant treatment outcomes. The median hospital stay was 14 days for TA repair and 10 days for TAA repair (p = 0.057). The hospital, 30-day, and 90-day mortality rates were 17.3%, 26.9%, and 34.6%, respectively, for TA repair and 4.6%, 8.2%, and 10.1%, respectively, for TAA repair (p < 0.001). The rate of stroke and transient ischemic attack was significantly higher following TA repair (7.7% vs. 1.2%, p = 0.002).
The annual number of inpatient treatment cases is continuously rising
In total, 40 patients (7.7% in the TA group and 3.9% in the TAA group, p = 0.319) were transferred to another hospital following treatment. Hospital readmission was necessary in the further course in 3.8% and 2.7% of patients, respectively, while repeat surgery was performed in 86.5% and 75.5% of patients, respectively.
A continuous rise was seen throughout the study period in the annual number of cases of inpatient treatment (from 7 in 2008 to 201 in 2016; proportionately 75 to April 2017). This corresponds to an absolute increase of more than 2800% between 2008 and 2016 and 283% between 2010 and 2016 (Fig. 3).
Discussion
This large-scale German analysis of claims data on complex endovascular repair of aortic diseases is the first study to analyze a database that provides an insight into the actual situation in terms of multicenter care. It demonstrates that there are significant differences between TA and TAA care in terms of age and gender distribution, short-term and medium-term mortality, and complications.
The endovascular treatment of aortic diseases that may involve visceral or supra-aortic vessels remains a challenge in modern vascular surgery. The evidence available on risk factors and treatment outcomes is largely based on single-center case series (Table 4). Due to possible selection and publication bias, as well as the unknown external and internal validity of these data, a comparison of the results with large registry or claims data is useful. On the whole, the patient characteristics and endpoints of the published case series of 1569 patients from 8 single center studies vary considerably. While the technical success in the case series was consistently high at 92–100%, the 30-day mortality rate among patients treated between 2001 and 2016 was between 0% and 6.2%. There were also marked differences between the respective cohorts included in terms of patient age (70.5–75 years) and the proportion of male patients (47–93.8%) (Table 4). These differences make it likely that relevant confounders were present. Mastracci et al. (610 type IV thoracoabdominal aortic aneurysms, TAAA) [22] and Eagleton et al. (354 type II and type III TAAA) [13] published the results of the largest study in terms of numbers with the longest post-interventional follow-up. Technical success was 97% and 94.1%, respectively, with a 30-day mortality rate of 4.8% for type II to type III TAAA. Aneurysm-related mortality was as low as 2% at 8 years following type IV TAAA repair. A total of 18.6% of patients with type II to type III TAAA had pre-existing kidney failure. Acute kidney failure was detected following intervention in 5.1% of patients and permanent spinal ischemia in 4% [13]. The results of 100 cases of consecutive endovascular repair of complex abdominal aortic aneurysms (AAA, including iliac findings) and TAAA were reported in the most recent prospective single center analysis by Schanzer et al. [28]. The average hospital stay in this case series was only 3.6 days. At 30 days, 3% of patients had died and intestinal ischemia was seen in 1% of cases. Paralysis, heart attack, and stroke were not observed [28]. Another single center analysis conducted by Budtz-Lilly et al. demonstrated a 30-day mortality rate of 2.8% and a 90-day mortality rate of 9.9% based on the retrospective data of 71 consecutively treated patients. In all, 15.0% (juxtarenal AAA) and 22.6% (TAAA) of patients had chronic kidney failure prior to intervention. Permanent post-procedural spinal damage was observed in only 2.8% of patients [9].
Our current analysis of claims data cannot readily confirm the results of the abovenamed single center analyses and case series. A possible selection bias is already evident in terms of the age and gender distribution. Patients in the single center trials were somewhat older and, with one exception, more frequently male compared with this study population. Closer scrutiny of the disparately defined comorbidities in the various study populations revealed other relevant differences. Whereas there is acceptable concordance in the rates of diabetes, cardiac arrhythmia, and chronic kidney disease between the different studies, significant differences are seen particularly in peripheral vascular diseases (e. g., peripheral arterial occlusive disease, coronary heart disease, and carotid stenosis). For example, chronic obstructive pulmonary diseases (COPD) are significantly more rarely coded in the DAK database compared with the primary data sources (Table 5). The validity of data from non-quality assured registries and claims data sources has recently been the subject of regular controversy [8, 32]. Projects designed to validate the data are also limited due to differing definitions of data collection parameters. In this context, the use of the Elixhauser comorbidity classification (into a total of 30 different groups) in this study improves comparability between different administrative records and WHO coding systems [14, 15, 25].
A further limitation in terms of valid comparability arises from the studies’ different inclusion periods. The question of whether improved generations of products, the introduction of new procedures and techniques, and the individual interventionalist’s learning curve as possible influencing factors has long been discussed [10, 20]. If one looks at the marked rise in the annual number of cases (Fig. 3), it becomes apparent that the reality of nationwide medical care in 2010, with around one third of today’s annual case numbers, cannot be easily compared across the board with the situation in 2017. To this one can add the rising number of previously treated patients in whom a higher rate of post-interventional complications (e. g., spinal ischemia) can be expected.
The treatment reality from 2010 is not comparable with the situation in 2017
Against this background, the question arises as to which criteria can be used to obtain informed consent from suitable patients and which information can be passed on to patients in an evidence-based manner. Since single center analyses that lack independent data monitoring and validation tend to have system-related selection and publication biases, independent sources of data are required in order to make comparisons with the reality of nationwide medical care. Although claims data can possibly close this gap, they in turn are subject to relevant limitations.
Limitations
Since DAK-G claims data are primarily collected for administrative and reimbursement purposes, conscientious data validation and quality assurance is required for their secondary use [6]. Internal validity varies and is generally greater for reimbursement-relevant codes than for codes that are not relevant to reimbursement. In the meantime, study projects such as the VISION initiative in the USA or the IDOMENEO study in Germany are addressing in greater detail the validity of claims data in vascular outcome assessment and treatment research [5]. Due to their better external validity compared with registry data, claims data are also suitable for analyzing rare events or treatments, such as in complex aortic pathologies. In contrast to registry surveys, where the treating physician often decides which data are submitted, the collection of claims data is not limited to isolated diseases, individual specialist disciplines or the duration of hospital stay. Particularly in the case of group comparisons, one can also assume that so-called overcoding for reimbursement reasons occurs in both groups to the same extent; as such, the results obtained could still be valid. Naturally, the analysis of claims data cannot replace randomized controlled trials (RCT); however, collecting supplementary data and comparing RCTs with the reality of medical care can provide important insights.
Conclusion
This large-scale analysis of claims data to demonstrate the actual situation in multicenter care revealed relevant differences not only in terms of patient age, gender, and mortality between the groups analyzed (TA vs. TAA), but also in comparison with the study results currently available. The significantly higher stroke rate in complex endovascular TA repair is also worthy of note. Multicenter, validated registry studies to compare primary and secondary data sources are recommended.
Change history
16 August 2018
Erratum to:
Gefässchirurgie (2018)
https://doi.org/10.1007/s00772-018-0387-7
The article was wrongly published under the article type “Review”. Please note that the article is an “Original Paper”.
The publisher apologizes to the authors and …
References
Augustin M, Anastasiadou Z, Schaarschmidt ML et al (2016) Versorgung des Hautkrebses in Deutschland. Hautarzt 67:544–548
Behrendt C‑A, Debus ES, Wipper S et al (2017) Das thorakoabdominelle Aortenaneurysma. Hamb Arztebl 71:12–16
Behrendt C‑A, Pridöhl H, Schaar K et al (2017) Klinische Register im 21. Jahrhundert – Ein Spagat zwischen Datenschutz und Machbarkeit? Chirurg 88(11):944–949. https://doi.org/10.1007/s00104-017-0542-9
Behrendt CA, Tsilimparis N, Diener H et al (2014) Einführung des GermanVasc. Gefasschirurgie 19:403–411
Behrendt CA, Härter M, Kriston L et al (2017) IDOMENEO – Ist die Versorgungsrealität in der Gefäßmedizin Leitlinien- und Versorgungsgerecht? Gefasschirurgie 22:41–47
Behrendt CA, Heidemann F, Riess HC et al (2017) Registry and health insurance claims data in vascular research and quality improvement. Vasa 46:11–15
Behrendt CA, Sedrakyan A, Riess HC et al (2017) Short-term and long-term results of endovascular and open repair of abdominal aortic aneurysms in Germany. J Vasc Surg 66(6):1704–1711.e3. https://doi.org/10.1016/j.jvs.2017.04.040
Björck M, Mani K (2017) Publication of vascular surgical registry data: strengths and limitations. Eur J Vasc Endovasc Surg 54(6):788. https://doi.org/10.1016/j.ejvs.2017.09.013
Budtz-Lilly J, Wanhainen A, Eriksson J et al (2017) Adapting to a total endovascular approach for complex aortic aneurysm repair: outcomes after fenestrated and branched endovascular aortic repair. J Vasc Surg 66(5):1349–1356. https://doi.org/10.1016/j.jvs.2017.03.422
Budtz-Lilly J, Björck M, Venermo M, Debus ES, Behrendt CA, Altreuther M, Beiles B, Szeberin Z, Eldrup N, Danielsson G, Thomson I, Wigger P, Khashram M, Loftus I, Mani K (2018) The Impact of Centralisation and Endovascular Aneurysm Repair on Treatment of Ruptured Abdominal Aortic Aneurysms Based on International Registries. Eur J Vasc Endovasc Surg (in press)
Coselli JS, Bozinovski J, Lemaire SA (2007) Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 83:S862–S864 (discussion S890–862)
Destatis SB (2014) Krankenhausdiagnosestatistik. In: Statistisches Bundesamt DeStatis (Gesundheitsberichterstattung des Bundes)
Eagleton MJ, Follansbee M, Wolski K et al (2016) Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg 63:930–942
Elixhauser A, Halpern M, Schmier J et al (1998) Health care CBA and CEA from 1991 to 1996: an updated bibliography. Med Care 36:MS1–MS9, MS18–147
Elixhauser A, Steiner C, Harris DR et al (1998) Comorbidity measures for use with administrative data. Med Care 36:8–27
Etheredge SN, Yee J, Smith JV et al (1955) Successful resection of a large aneurysm of the upper abdominal aorta and replacement with homograft. Surgery 38:1071–1081
Fischer F, Hoffmann K, Mönter N et al (2013) Kostenevaluation eines Modells der Integrierten Versorgung für schwer psychisch Kranke. Gesundheitswesen 76:86–95
Grimme FA, Zeebregts CJ, Verhoeven EL et al (2014) Visceral stent patency in fenestrated stent grafting for abdominal aortic aneurysm repair. J Vasc Surg 59:298–306
Kristmundsson T, Sonesson B, Dias N et al (2014) Outcomes of fenestrated endovascular repair of juxtarenal aortic aneurysm. J Vasc Surg 59:115–120
Martin-Gonzalez T, Mastracci TM (2017) Learning curve in fenestrated and branched grafting. J Cardiovasc Surg 58:261–263
Martin-Gonzalez T, Pincon C, Hertault A et al (2015) Renal outcomes analysis after endovascular and open aortic aneurysm repair. J Vasc Surg 62:569–577
Mastracci TM, Eagleton MJ, Kuramochi Y et al (2015) Twelve-year results of fenestrated endografts for juxtarenal and group IV thoracoabdominal aneurysms. J Vasc Surg 61:355–364
Müller I, Freitag MH, Poggensee G et al (2012) Evaluating frequency, diagnostic quality, and cost of lyme borreliosis testing in Germany: a retrospective model analysis. Clin Dev Immunol 2012:1–13
Piffaretti G, Fontana F, Franchin M et al (2017) Total endovascular treatment for extent type 1 and 5 thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 154:1487–1496.e1
Quan H, Sundararajan V, Halfon P et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–1139
Safi HJ, Miller CC 3rd, Carr C et al (1998) Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg 27:58–66 (discussion 66–58)
Scali ST, Goodney PP, Walsh DB et al (2011) National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg 53:1499–1505
Schanzer A, Simons JP, Flahive J et al (2017) Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg 66(3):687–694. https://doi.org/10.1016/j.jvs.2016.12.111
Sidloff D, Stather P, Dattani N et al (2014) Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation 129:747–753
Stoberock K, Rieß HC, Debus ES, Schwaneberg T, Kölbel T, Behrendt CA (2018) Gender differences in abdominal aortic aneurysms in Germany using health insurance claims data. Vasa 47:36–42
Van Walraven C, Austin PC, Jennings A et al (2009) A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 47:626–633
Venermo M, Mani K, Kolh P (2017) The quality of a registry based study depends on the quality of the data – without validation, it is questionable. Eur J Vasc Endovasc Surg 53:611–612
Acknowledgements
All analyses were carried out by GermanVasc, which is an interdisciplinary university working group that was founded in Hamburg in 2014 [4] and which is concerned with the data protection compliant [3] use of registry and routine data in vascular treatment research and quality development [6]. We would like to thank the German Association for the Promotion of Science and Humanities (Stifterverband für die Deutsche Wissenschaft e. V.) for making this study possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.-A. Behrendt, H. C. Rieß, T. Schwaneberg, F. Heidemann, N. Tsilimparis, A.-A. Larena-Avellaneda, H. Diener, T. Kölbel and E.S. Debus declare that they haves no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
The authors C.-A. Behrendt and H.C. Rieß share first authorship.
The authors T. Kölbel and E.S. Debus share last authorship.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Behrendt, CA., Rieß, H.C., Schwaneberg, T. et al. Complex endovascular treatment of intact aortic aneurysms. Gefässchirurgie 23 (Suppl 1), 32–38 (2018). https://doi.org/10.1007/s00772-018-0387-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00772-018-0387-7