Abstract
An overview of the current state of knowledge on the pollution of agricultural soils with microplastic and nanoplastic (MnP) particles is provided and the main MnP sources are discussed. MnP transport mechanisms from soil to groundwater, as well as the potential impact of MnPs on soil structure are considered, and the relevance of co-contaminants such as agrochemicals is further highlighted. We elaborate on why MnPs in soil and groundwater are understudied and how analytical capabilities are critical for furthering this crucial research area. We point out that plastic fragmentation in soils can generate secondary MnPs, and that these smaller particles potentially migrate into aquifers. The transport of MnP in soils and groundwater and their migration and fate are still poorly understood. Higher MnP concentrations in agricultural soils can influence the sorption behavior of agrochemicals onto soil grains while attachment/detachment of MnPs onto soil grains and MnP-agrochemical interactions can potentially lead to enhanced transport of both MnP particles and agrochemicals towards underlying groundwater systems.
Zusammenfassung
In diesem Artikel liefern wir einen Überblick über den derzeitigen Kenntnisstand zu Mikro- und Nanoplastikpartikeln (MnP) in landwirtschaftlich genutzten Böden einschließlich der wichtigsten Quellen. Die Transportmechanismen und Auswirkungen von MnP in Böden sowie mögliche Eintragspfade ins Grundwasser werden ebenso betrachtet wie die Bedeutung von Co-Kontaminanten. Wir erläutern, warum MnP in Boden und Grundwasser unbedingt weiterer Forschung bedarf und weisen darauf hin, dass durch die Fragmentierung von Plastik sekundäre MnP entstehen, und dass diese kleineren Partikel potenziell in Grundwasserleiter transportiert werden können. MnP in landwirtschaftlich genutzten Böden können das Sorptionsverhalten von Agrochemikalien verändern und die unterschiedliche Bindung/Ablösung von MnP an Bodenkörner und die Wechselwirkungen zwischen MnP und Agrochemikalien können möglicherweise zu einem verstärkten Eintrag von MnP und Agrochemikalien in Grundwassersysteme führen. Es zeigt sich im Allgemeinen, dass der Transport von MnP in Böden und Grundwasser nur unzureichend bekannt ist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global plastic pollution has received growing attention as plastic waste has become an increasing environmental threat (Borrelle et al. 2020; Krause et al. 2021). Global annual plastic production currently surpasses more than 450 million metric tons (MMT), and is assumed to further increase by a factor of 2–3 by 2060 if no mitigating actions are taken (OECD 2022). With growing production, plastic waste has also increased to an estimated 353 MMT in 2019 (OECD 2022) with about 22 MMT having leaked into the environment as mismanaged plastic waste. Mitigation of such waste will thus be a major challenge for decades to come (Borrelle et al. 2020).
Plastic debris is frequently categorised into subgroups based on particle size (Galgani et al. 2013; Alimi et al. 2018), distinguishing between macroplastics (> 25 mm), mesoplastics (5–25 mm), microplastics (1 µm–5 mm) and nanoplastics (< 1 μm). However, these individual size ranges are not unanimously established but often serve as mere indicators and a basis for further discussion (see Hartmann et al. (2019) for a review). Microplastic and nanoplastic particles (MnPs) are typically classified as primary or secondary particles. Primary MnP particles are intentionally manufactured in their size range, e.g., for use in cosmetics, personal care products, industrial processing, textile applications, or synthetic clothes production (Gregory 1996; Browne et al. 2011). In addition, secondary MnPs can result from the weathering and fragmentation of larger plastic items. Both primary and secondary MnPs can enter the environment via a variety of point sources (e.g., wastewater treatment plants) and diffuse sources (e.g., urban and agricultural runoff) (Waldschläger et al. 2020) as will be discussed in the following chapters.
MnPs have been detected in all environments, but their versatility and omnipresence make source identification very challenging (Rochman et al. 2019; Horton et al. 2017). A substantial number of studies have been dedicated to MnPs in marine (Kane and Clare 2019; Nel et al. 2020; Alfaro-Núñez et al. 2021) and riverine environments (Li et al. 2018a; Hoellein et al. 2019; Drummond et al. 2022) where MnPs have been found to have the capability to enter food webs (Krause et al. 2021) or pose a potential threat to a variety of species (Kukkola et al. 2021; Kwak et al. 2022) and even entire ecosystems (de Souza Machado et al. 2018). However, research on the fate, transport and impact of microplastics in soils and especially groundwater is still in its infancy (Re 2019; Goeppert and Goldscheider 2021). While microplastics were recently found in drinking water (Eerkes-Medrano and Thompson 2018; Schymanski et al. 2018; Koelmans et al. 2019) the occurrence, fate and major transport processes of MnPs in soil and groundwater are far less understood.
Concerns have been raised with respect to the techniques used in the agricultural sector that may cause MnP pollution in agricultural soils (Kumar et al. 2020; Tian et al. 2022). Common agricultural practices such as the use of plastic films for insulation and mulching, plastic water pipes for irrigation and plastic greenhouse covers, or the application of sewage sludge as fertilizer, are some of the potential sources of MnP contamination (Steinmetz et al. 2016; Brodhagen et al. 2017) in agricultural areas. Unsurprisingly, agricultural soils are therefore estimated to receive a major portion (up to 14%) of the total released plastic to the environment (Alimi et al. 2018; Horton et al. 2017) and are thought to be a major sink for MnP pollution, with the number of MnPs released onto land estimated to be 4–23 times more than to the ocean (Horton et al. 2017).
Whether agricultural soils are mostly permanent sinks for MnPs or can also act as an intermediate storage site on their way to nearby freshwater systems and the groundwater is much debated (The Royal Society 2019) but first studies indicate that in general, transport of MnPs in the subsurface is potentially taking place (Viaroli et al. 2022; Goeppert and Goldscheider 2021). On the one hand, soil erosion by wind and surface runoff represents a key mechanism of MnP transport to nearby rivers or lakes similar to fine particulate organic matter from agriculture to surface water (Schönenberger et al. 2022). Additionally, MnPs in agricultural soils can be transported further by drainage water and over time might be able to reach nearby water bodies via surface runoff or groundwater recharge. Yet, surprisingly little is known about actual MnP transport processes and pathways from agricultural topsoil, their retention times in the vadose and saturated zones, their potential for releasing chemical additives into the environment, their interaction with soil constituents such as organic matter, biofilms and the wider soil microbiome, or their co-transport to and within aquifers in the presence of nutrients and pesticides (Wanner 2021). Closing this knowledge gap seems imperative as the vadose zone, aquifers and nearby receiving freshwater bodies are inextricably linked and MnPs have the potential to migrate in pristine or weathered form between those compartments (Horton et al. 2017).
In this review, we provide an overview of the current understanding of MnP in agricultural soil and discuss the main sources and entry points into soils. We furthermore consider the potential transport mechanisms of MnPs from soil to groundwater, as well as the potential impact MnPs can have on soil structure and characteristics which could influence transport, and we discuss the relevance of possible co-contaminants such as pesticides. Finally, we elaborate on why MnPs in soil and groundwater are less studied than in marine and riverine systems and how analysis and detection capabilities are critical to furthering this research area.
Sources and amounts of micro and nanoplastics in agriculture areas
Plastic is prevalent in agriculture crop and livestock production because of its low cost, versatility and durability that make it a highly useful material for a wide range of different applications. In 2020, agricultural production systems accounted for 3.2% of European plastic demand, which represents around 1.5 MMT of plastic (PlasticsEurope 2019). Global plastic usage in agriculture is not well documented, but the FAO (2021) has estimated that at least 12.5 MMT of plastics are used globally, with 10 MMT of this attributed to crop and livestock production. Much of the plastic used in agriculture is of single use, that, if mismanaged, is at risk to persist in the environment and degrade into smaller particles over time. Estimates of MnPs in soils are limited, with only very few studies providing particle numbers or plastic mass due to the lack of analytical methods capable of readily and cost-effectively identifying and quantifying microplastics in soils, in particular smaller fractions below 5–10 µm (Caputo et al. 2021). Büks and Kaupenjohann (2020) provide a comprehensive meta study on MnPs in different soils comparing agricultural and horticultural sites around the world. They found microplastic concentrations in agricultural soils to be between < 1 and < 530,000 particles kg−1 dry weight (based on 118 samples) with average concentrations of about 1200 particles kg−1. Concentrations seem highly variable and strongly depend on the approaches used for sampling, polymer extraction and identification.
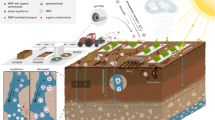
Plastic enters agricultural soils in all size ranges with pathways varying based on agricultural practices, urban influence, and hydrometeorological drivers (Fig. 1). Microplastic addition can be intentional and unintentional. Unintentional microplastic addition is rooted in the fragmentation of larger plastics, for example when using plastic mulching film or plastic packaging. Other unintentional sources of microplastics include atmospheric deposition, machine and tire wear particles from agricultural vehicles and machinery, flooding events, or managed aquifer recharge. Microplastics are also directly applied to agricultural soil by using sewage sludge or other compost as fertiliser, by irrigation with wastewater, and through the application of agrochemicals and seeds which are often encapsulated in a polymer coating (Yang et al. 2021; Tian et al. 2022).
Current agricultural practices result in significant emissions of MnPs to soils. Plastic mulch films that are heavily used in agriculture to improve crop yields (Qian et al. 2018) and water consumption efficiency account for about 75% of all plastic usage in agriculture (APE Europe 2019).
Plastic mulch covers about 20 million hectares of farmland globally, with China accounting for 90% of this area (Yang et al. 2021). Research in China has found high levels of microplastic pollution with 40.35 mg kg−1 soil in areas where plastic mulching had been used continuously for 30 years (Li et al. 2020). According to Ramos et al. (2015), plastic mulch is often made of low-density polyethylene (LDPE) and removal/disposal is difficult, so usually it remains in situ, resulting in fragmentation to smaller MnPs in soil over time. Biodegradable plastic mulches are increasingly being suggested as alternatives, yet these will also form microplastics, thus further contributing to the pollution issue (Serrano-Ruiz et al. 2021). Moreover, biodegradable particles are assumed to degrade over a comparably short time span with potentially no ecotoxicological implications. Thus, polymer degradability is not the only factor to consider while attempting to replace common-use plastics with biodegradable products. The potential adverse effects of plastic additives and their degradation products on biota also need further evaluation (Bettas Ardisson et al. 2014; Haider et al. 2019; Palsikowski et al. 2018). Unfortunately, ecotoxicological data for biodegradable polymers is scarce (Haider et al. 2019).
Additional problems arise from those plastic films that contain phthalate esters or other chemical additives that have been shown to adversely affect microbial community composition and soil enzymatic activity (Liu et al. 2014; Qian et al. 2018).
The use of sewage sludge from wastewater treatment plants (WWTPs) has been estimated to be one of the largest sources of MnPs to agricultural soil (Hurley and Nizzetto 2018; Zubris and Richards 2005). While removal efficiency of WWTPs depends on their treatment process design, studies (Iyare et al. 2020; Gatidou et al. 2019) have shown that often more than 90% of microplastic influent concentrations are removed by WWTPs containing at least a secondary (biological) treatment stage. Here MnPs will accumulate in the sewage sludge which in many countries is applied as agricultural fertiliser as it has a positive impact on soil fertility (Corradini et al. 2019; Coors et al. 2016). However, the application of sewage sludge as fertiliser bears the risk of MnP contamination of agricultural soils. For example, sewage sludge from 28 WWTPs in China was found to contain average microplastic concentrations of 22.7 ± 12.1 × 103 particles kg−1 dry sludge (Li et al. 2018b). Microplastics have furthermore been found in other organic fertilisers such as composts (Bläsing and Amelung 2018).
MnPs can also be added to agricultural soil intentionally, for instance via the application of industrially produced chemical fertilisers and pesticides which often are encapsulated in a polymer shell (Puoci et al. 2008; Weithmann et al. 2018; Wang et al. 2019). For example, a recent study from Japan has shown that microcapsules from coated fertiliser accumulated in paddy fields with an average concentration of 144 mg kg−1 of soil (Katsumi et al. 2021). Furthermore, Accinelli et al. (2018, 2019) discussed more novel applications where crop seeds are directly laced with polymer films containing pesticides, binding agents and synthetic pigments and how these films can fragment through abrasion.
Wear and tear of agricultural equipment can also be considered a source of MnPs in agricultural soils. This includes tire wear abrasion, or abrasion of other machine parts made of plastics during machine operation. Additionally, tire wear can potentially accumulate in agricultural areas from nearby road-runoff and via atmospheric deposition as the wear products often constitute small particles in the low micrometre to nanometre range. For example, Kole et al. (2017) estimated that in the Netherlands, 67% of tire particles that are released to the environment, end up in the soil, making this a potentially large source of soil MnPs.
Agricultural soils also receive MnP input via direct atmospheric deposition. In fact, MnPs are omnipresent in the atmosphere and have been found even in remote locations such as the Pyrenees where Allen et al. (2019) encountered average daily deposition rates of 365 ± 69 particles m−2 d−1 while Kernchen et al. (2022) estimated daily deposition rates of 99 ± 85 particles m−2 d−1 for the River Weser catchment.
Another MnP source to agricultural soil is irrigation with both freshwater and wastewater depending on irrigation and drainage network design (Bläsing and Amelung 2018; Yang et al. 2021). However, comprehensive studies quantifying this potential MnP source to agricultural soils are still lacking. Irrigation practices differ widely but areas that extensively use potentially polluted surface water for irrigation such as on floodplains or terrace farms, or apply untreated municipal wastewater, might be especially prone to increased microplastic pollution. Additionally, natural events such as floods can result in microplastic addition to soils near rivers. For example, Scheurer and Bigalke (2018) found that 90% of 29 investigated Swiss floodplain soils in nature reserves contained microplastics.
MnPs accumulating in agricultural soil can potentially be transported further downwards over time into underlying groundwater and could therefore potentially be present as an emerging pollutant, especially in unconfined and shallow aquifers. Additionally, direct input of MnPs into aquifers under agricultural areas has also been recently highlighted. For example, Re (2019) and Viaroli et al. (2022) discuss MnP input due to practices such as managed aquifer recharge or the use of water abstraction near rivers with significant microplastic loads and groundwater-surface water interaction. In particular, rivers and streams are a primary transport vector for MnPs, which can infiltrate into streambed sediment up to a depth of twice the bedform amplitude (Boos et al. 2021) and accumulate there over time while posing a potential threat to freshwater resources. While Goeppert and Goldscheider (2021) show that transport of microplastics in alluvial aquifers over larger distances is possible, quantitative studies regarding the direct input of MnP through groundwater management practices are still lacking. Landfills, dump sites and domestic septic tank effluent might constitute further potentially significant local sources of MnP input into nearby soil and groundwater. The latter can be especially important in more sparsely populated areas without centralised wastewater treatment and with shallow water tables. While recent studies have demonstrated the potential of soil and groundwater to receive dissolved pollutants such as nutrients or artificial sweeteners from septic tank-based effluent (Tamang et al. 2022; Oldfield et al. 2020), studies focused on MnPs have yet to be conducted. The aforementioned input and vectors of MnPs into aquifers are important because the presence of plastic particles has been confirmed in source waters of several drinking water treatment plants, raising concern whether current conventional treatment technologies can satisfactorily remove plastic particles (Pulido-Reyes et al. 2019). For instance, Pulido-Reyes et al. (2019) show that ozonation, often applied in WWTPs in Europe, does not appear to either fragment plastic particles or change their aggregation state and does not affect MnP retention during water treatment. However, as an important result to ensure drinking water safety, Pulido-Reyes et al. (2022) also show that slow sand filtration during drinking water treatment is an efficient process for MnP removal.
MnP transport and fate in porous media
Soil and vadose zone
To better understand MnP transport and fate in soils and the vadose zone, previous studies have used lab-based column experiments where the soil or other media (e.g., glass beads) were well characterized and MnPs were typically spherical (e.g., Ron et al. 2019; Dong et al. 2018; Torkzaban et al. 2008; Keller et al. 2019). In those studies, it was demonstrated that MnPs can in principle migrate through soil and unsaturated porous media and pose a threat to soil and groundwater, even though both plastic particles as well as the porous media only mimicked natural conditions. Aggregation and deposition of MnPs as well as the water saturation seem to be important parameters that control MnP transport apart from physical properties of the porous media such as porosity and plastic size in some of these experiments (e.g. Hoggan et al. 2016, Keller et al. 2019, Sajjad et al. 2022).
Plastic particle size is especially critical as particles larger than pore throat openings found in the porous medium might simply accumulate in the uppermost part of the soil unless preferential flow paths or macropores exist (e.g., Bläsing and Amelung (2018)). On the other hand, the transport of MnP particles smaller than pore throat openings is mainly governed by the interaction with soil grains that act as collector surfaces (Liang et al. 2022). As with other engineered nanoparticles, interaction energies between these MnPs and the solid-water interface then play a decisive role, together with surface roughness and charge heterogeneity, on particle behavior (Adrian et al. 2018; Bradford et al. 2017). In a recent study, Keller et al. (2019) also showed that particle shape is an important parameter controlling MnP transport, with fibres being retained in soils to a much higher degree than spheres with a rough surface structure. They hypothesise that particle shapes that deviate strongly from a spherical form more often lose their orientation along flow lines.
Earthworms, microarthropods and other bioturbators can accelerate vertical transport of MnPs by creating preferential flow paths (Heinze et al. 2021). Some studies have shown that this can lead to MnPs being transported to depths of up to 40–50 cm (Huerta Lwanga et al. 2017; Rillig et al. 2017; Rodríguez-Seijo and Pereira 2019). Depending on the depth of the water table, these MnPs could thus reach shallow aquifer systems. Root growth and microbial communities can further affect soil structure (e.g., by creating vertical macropores) and therefore MnP transport (Huerta Lwanga et al. 2017; Prata et al. 2021; Rillig et al. 2017). Yet other studies have also revealed MnP transport in the absence of earthworm and microarthropods (Yan et al. 2020). For example, apart from biological activity, weather conditions such as wet-dry cycles with associated soil cracks can also accelerate vertical MnP transport (O’Connor et al. 2019).
Soil typically contains some degree of organic matter and studies on MnP transport using natural soils (Huerta Lwanga et al. 2017; Yan et al. 2020; Keller et al. 2019; Rillig et al. 2017; Rodríguez-Seijo and Pereira 2019), have shown a tendency for particles to attach onto mobile organic matter, resulting in accelerated MnP transport. Other studies have found MnP transport to simply increase with increasing organic matter content (Alimi et al. 2021; Keller et al. 2019; Yan et al. 2020).
Depending on the respective MnP properties, plastic particles might also be a favorable environment for microorganisms that attach to the polymer surfaces and degrade MnP by secreting enzymes in order to obtain energy for their growth (Danso et al. 2019). This process, paired with photo-oxidation, can generate secondary MnPs, which in turn are more easily transported to greater depths (Piehl et al. 2018) towards the groundwater (Fig. 2). Agricultural practices can also affect the distribution of MnPs within soils. For instance, conventional tillage practices such as harvesting or plowing could turn over the surface and deep soil, resulting in plastic from topsoils being transported to greater depths.
Plastic fragmentation leading to secondary MPs and NPs. Due to their smaller size, plastic might migrate through soils into underlying aquifers
Plastikfragmentierung, die zu sekundären MP und NP führt. Aufgrund der geringeren Größe kann Plastik durch den Boden in darunterliegende Grundwasserleiter migrieren
MnP accumulation in the soil can directly affect soil physico-chemical properties (Zhang et al. 2019; Dong et al. 2021a; Lozano et al. 2021) and changes to soil structure and composition are important in that they will affect further MnP transport. For example, Wang et al. (2020) showed that soil bulk density, water holding capacity and soil structure were affected by MnP concentration. Cramer et al. (2022) showed that for areas with high MP content, water infiltration and thus transport were significantly reduced and decreasing water saturation paired with entrapped air could lead to more tortuous flow paths. MnP-induced changes in available pore space (e.g., reduction in pore size due to filling of pore spaces with MnPs) have been shown to lead to both enhanced or reduced MnP transport (Scheurer and Bigalke 2018; Zhang et al. 2019). According to Ingraffia et al. (2022) the effects of plastic on soil parameters and soil erosion are strongly dependent on soil type.
Chemical conditions in soils also affect the transport and retention of MnPs. For example, enhanced MnP transport was observed with increasing pH (Jiang et al. 2021; Wu et al. 2020) and decreasing ionic strength (Dong et al. 2018; Hou et al. 2020; Wu et al. 2020). While smaller plastic particles (0.02 µm) do not show a change in transport in the presence of iron oxides, the transport of larger particles seems to be affected. In addition, an increase in MnP transport with decreasing Fe/Al-oxide contents has also previously been observed (Li et al. 2019; Tong et al. 2020; Wu et al. 2020). Moreover, ionic compounds show an impact on MnP transport. Generally, certain compounds such as KCl and CaCl2 are more effective in decreasing plastic mobility in porous media than others such as NaCl and MgCl2 (Jiang et al. 2021; Yan et al. 2020). Overall, cations with smaller ionic radii (Na+ and Mg2+) seem to have greater hydrated radii, which can result in weaker charge screening and obstruction of deposition through steric hindrance, which can thus decrease MnP retention in porous media.
Groundwater
To date only few studies exist that assess MnP contamination of aquifers, with none of them focusing specifically on groundwater resources underlying major agricultural areas (see Viaroli et al. (2022) for a review). Average MnP concentrations in groundwater vary from less than 0.001 particles l−1 (Johnson et al. 2020) for a chalk and sandstone aquifer in the UK, to about 7 particles l−1 in a karst groundwater system in the US (Panno et al. 2019), to 38 particles l−1 in an alluvial unconfined aquifer in Australia (Samandra et al. 2022) and even 2103 particles l−1 in China (Mu et al. 2022). This wide range of concentration highlights a dire need for further observations with regards to the occurrence of MnP in aquifers around the world using well established protocols for sampling, extraction and quantification to ensure better comparability among sites. Additionally, observed groundwater MnP concentrations can be influenced by poorly developed wells, as plastic particles can potentially enter the subsurface during well construction, pumping or cleaning. Moreover, practices such as managed aquifer recharge or the use of water abstraction near rivers can be a primary MnP transport vector. Because MnPs can accumulate in streambed sediment and subsequently enter aquifer systems (Frei et al. 2019), they pose a potential threat to drinking water supplies, especially during drinking water production via riverbank filtration (Boos et al. 2021; Frei et al. 2019; Gillefalk et al. 2018).
Similar to soils, fine-grained unconsolidated aquifer material typically contains organic matter, which is an important parameter controlling transport of colloidal particles (Adrian et al. 2019). Aquifer chemical properties such as pH or ionic strength can also significantly affect MnP transport and retention (Dong et al. 2021b, Jiang et al. 2021; Wu et al. 2020). In summary, MnP transport through subsurface porous media depends primarily on i) MnP properties such as size, density, shape, composition and surface characteristics; ii) environmental conditions of the porous medium such as grain size distribution and material composition, pore geometry, organic matter content, groundwater flow velocity, and groundwater or soil water chemistry, and iii) the interaction of MnPs with flora and fauna such as plant roots, biofilm formation or invertebrate activity. Even though transport of MnPs in soils has been observed, soils may under specific circumstances also represent effective barriers against groundwater plastic contamination. However, while a few studies show that MnPs can potentially reach groundwater and thus may pose a credible threat to water supplies, there is still little representative data on the transport of MnP particles in natural soil and groundwater environments. Also, many transport and fate processes such as MnP interactions with the porous medium or additive leaching over time are so far poorly understood. Additionally, analytical detection limits in environmental groundwater MnP studies might have resulted in an underestimation of plastic particles thus far.
Interaction between contaminants and MnPs
MnPs with high specific surface area and hydrophobicity are more likely to favour adhesion and sorption of organic contaminants. Hydrogen bonds, hydrophobic interactions, van der Waals forces and electrostatic interactions can lead to adhesion and sorption of organic contaminants on plastic particle surfaces (Atugoda et al. 2020). For example, Shi et al. (2022) found antibiotics in groundwater samples, where concentrations were significantly correlated with MP occurrences. This is important as due to their extensive application in agriculture, both pesticides as well as other agrochemicals and plastics co-exist. However, sorption affinity strongly differs for different agrochemical contaminants and plastic types, with Kd values potentially varying over several orders of magnitude (Wanner 2021). Furthermore, the sorption of pesticides to MnP particles depends on a number of parameters, such as hydrophobicity of the pesticide, the pH of the soil, the polymer type and the degree of weathering of the MnP (Hüffer et al. 2019; Seidensticker et al. 2018).
Moreover, microbial colonization can have a significant impact on agrochemical contaminant-plastic interaction. Puglisi et al. (2019) showed that biodegradable and non-biodegradable plastics can be colonized by microbial communities. It is likely that MnPs provide favorable habitats for different microorganisms, leading to biofilm build-up. Biofilm and consequently microorganism activity can lead to the mobilisation of pesticides or other agrochemical contaminants. Thus, long-distance migration of contaminants and the production of metabolites in some cases with greater toxicity than the parent component are possible (Li et al. 2018b). For example, Hüffer et al. (2019) observed enhanced transport of atrazine and 4‑(2,4-dichlorophenoxy) butyric acid through plastic-containing agricultural soils as compared to plastic-free soils. In contrast, Castan et al. (2021) indicate that desorption of most organic contaminants is too fast for MnPs to act as transport facilitators in soils, and contaminant transport enabled by MP was found to be relevant only for very hydrophobic contaminants (log Kow > 5) under preferential flow conditions. Thus, their study suggests that MnPs do not significantly enhance contaminant mobility.
In summary, whether and to what extent MnPs facilitate the transport of organic contaminants in soil remains uncertain. Plastic-induced changes in the sorption behavior of pesticides to agricultural soils can enhance pesticide transport twofold: through plastic particle-mediated pesticide transport or through a reduced soil sorption capacity depending on the sorption affinity of the pesticide to the plastic compared to natural soil particles (Fig. 3).
Plastic-induced change of the transport and sorption behavior of agrochemicals such as pesticides to agricultural soils. a Enhanced transport because plastic particles mediate pesticide transport and through a reduced soil sorption capacity depending on the sorption affinity of pesticide to plastic compared to natural soil particles. b Microorganism activity can lead to pesticide or other agrochemical contaminant degradation and sorption and thus to reduced transport but metabolisation can still occur
Plastikbedingte Veränderung des Transport- und Sorptionsverhaltens von Agrochemikalien (z. B. Pestiziden) in landwirtschaftlichen Böden. a Verstärkter Transport, da Plastikpartikel den Transport von Pestiziden beschleunigen und die Sorptionskapazität des Bodens je nach Sorptionsaffinität des Pestizids an Kunststoff im Vergleich zu natürlichen Bodenpartikeln verringert wird. b Die Aktivitäten von Mikroorganismen können zum Abbau und zur Sorption von Pestiziden oder anderen agrochemischen Schadstoffen führen und somit den Transport verringern, aber es kann auch eine Metabolisierung stattfinden
Although interactions of agrochemical contaminants (pesticides, fungicides, pharmaceuticals, etc.) and MnPs are of high relevance, very little is known about their preferential transport processes. Studies focussing on sorption mechanisms of agrochemical contaminants to plastic are scarce (Castan et al. 2021; Seidensticker et al. 2018; Hüffer et al. 2019; Wanner 2021). For small particles, for instance, attachment to collector surfaces (soil grains) happens not evenly around a grain but only in certain spots depending on surface roughness and local energy minima/maxima. Additionally, elastomeric nanoparticles can form chemical bonds with grains but when those potential bonding sites are already occupied (e.g., through pesticide sorption) nanoparticle transport through the subsurface can be enhanced with potentially much higher subsurface concentrations occurring. However, this possible behaviour is not well researched yet.
Analysis and detection capabilities
MnPs in groundwater have been rarely discussed, with only a few studies focusing on their occurrence (e.g., Table 1 in Khant and Kim (2022)). There are no standardized or well-established procedures for quantifying or even reporting MnPs in soil and groundwater samples, even though they are needed (Viaroli et al. 2022) and existing community efforts (e.g., Cowger et al. (2020)) show potential for designing more formal guidance documents. The scarcity of laboratory and especially field data with regard to the mobility of MnPs in agricultural soils and groundwater is strongly linked to analytical challenges around their quantification. Analytical methods for microplastics in soils have been reviewed extensively (e.g., Möller et al. 2020), with larger microplastics (≥ 500 µm) being relatively easily sorted and identified (Schrank et al. 2022). However, there is no consensus yet on the analysis of smaller microplastics (< 500 µm) (Möller et al. 2020). Generally, procedures followed to extract microplastics from soil samples will include a density separation step using a high-density salt solution that will separate out any inorganic materials (Qi et al. 2020). The separated MnP supernatant is then digested by means of acids, alkalis, oxidation, or enzymatic methods to remove any organic matter (although sometimes digestions are carried out before density separation). Once these steps are completed, most of the soil matrix should have been removed before polymer identification and analysis start.
Commonly used MnP detection and quantification methods can be grouped as follows, but they all have advantages and disadvantages:
-
Visual identification using light microscopy, which can be combined with fluorescent dyes such as Nile Red (Maes et al. 2017; Nel et al. 2021).
-
Vibrational spectroscopy such as Raman or Fourier transform infrared spectroscopy (microFTIR) (Käppler et al. 2016).
-
Chromatography such as pyrolysis gas chromatography mass spectrometry (Pyr GC-MS) (Fischer and Scholz-Böttcher 2017) or thermal extraction desorption gas chromatography mass spectrometry (TED GC-MS) (Dümichen et al. 2017).
-
Thermogravimetric analysis (David et al. 2018)
Light microscopy is a relatively low-cost and simple technique but is only suitable for the identification of larger plastic fragments, plus it does not provide information about polymer type. Combining light microscopy with the application of fluorescent dyes such as Nile Red can improve the accuracy of particle identification. However, as many dyes are unselective, organic matter such as chitin (Helmberger et al. 2020) needs to be properly digested before MnP quantification as it could otherwise exhibit a strong fluorescent signal itself. Any form of light microscopy of small particles can also potentially lead to observer bias and as such it should not be the only method of quantifying plastics in a sample. The more frequent use of vibrational spectroscopy methods is often restricted by limited access to the more expensive equipment as compared to light microscopy and thus often only a limited number of subsamples are analysed. The main benefits of Raman spectroscopy and microFTIR analyses are that they provide polymer identification, as well as information on MnP shape and size. FTIR is thought to be reliable at detecting microplastics down to 10–20 µm in size, whereas Raman can theoretically go down to 1 µm (Xu et al. 2019). Chromatography methods such as Pyr-GC-MS and TED-GC-MS, and thermogravimetric methods are good at detecting microplastics in environmental samples, and they can identify the polymer type. However, they are destructive methods so, while providing MnP and additives total mass, they are unable to provide information on number and size of particles.
Various attempts have been made to lower the detection limits of existing analytical methods to study nano-sized plastics. Examples are the coupling of Raman microscopy and field-flow fractionation enabled by optical tweezers (RM-FFF) (Schwaferts et al. 2020) as well as accelerated solvent extraction combined with quantitative proton nuclear magnetic resonance (q-1H NMR) (Nelson et al. 2019). Using the RM-FFF method, detection limits for nanoplastics are down to a size of ~200 nm at a minimum concentration of 1 mg l−1, however, the method was primarily developed for aqueous solutions and it remains to be seen whether it is also applicable to complex samples such as agricultural soils.
Every step during sampling and sample processing can introduce additional contamination with MnPs, but MnPs contained in the original sample can also get lost during transport, filtration or digestion. Hence, it should be common practice to establish both negative and positive controls (Koelmans et al. 2019; Philipp et al. 2022) and whenever possible, sampling in and use of plastic materials has to be avoided (Koelmans et al. 2019). The use of both methods and field blanks helps to verify the existence of potential contamination, while laboratory control samples account for losses.
Summary and conclusions
Current agricultural practices constitute potentially significant sources of plastic, with plastic entering agricultural soils in a wide size range and via numerous pathways. Plastic fragmentation can generate secondary MnPs that might migrate through soils into underlying aquifers analogously to natural colloids, although it has been argued that soils may still represent an effective barrier against groundwater contamination by plastics. So far, very few studies have investigated the transport of MnP particles in soils and groundwater and thus their migration and fate are poorly understood, leading to a large knowledge gap that must be addressed in order to provide adequate risk assessment and to develop appropriate management practices. Initial results indicate that higher MnP concentrations in agricultural soil can change the sorption behavior of agrochemicals (e.g., pesticides) onto soil grains. Varying attachment/detachment of MnPs onto soil grains and MnP-agrochemical interactions can then potentially lead to enhanced co-transport of both MnPs and agrochemicals towards underlying groundwater systems depending on chemical characteristics as well as MnP and agrochemical properties. This hints towards a contamination risk for aquifers and drinking water supplies underlying agricultural areas which would call for improved regulatory measures regarding plastic use in agriculture. However, further research is needed to better understand MnP-agrochemicals-soil interactions, and MnP fate and retention times in soil and groundwater.
Despite a general increase in MnP studies, open questions and challenges regarding the identification of MnP particles remain, especially when dealing with complex agricultural soils and groundwater samples. Future research needs can be broadly classified into six open topic areas: (1) the assessment of occurrence and distribution of MnPs, especially in groundwater; (2) understanding MnP transport mechanisms and pathways, and the identifying factors that influence their occurrence and distribution; (3) development of rapid quantification methods allowing for high sample throughput with low detection limits and yet sufficiently high accuracy and measurement repeatability; (4) standardization of sampling, processing and analysis procedures; (5) improved understanding of plastic-agrochemical interactions and relevance for groundwater quality, and finally (6) assessing the significance of the impact of plastics on environmental and human health and wellbeing.
References
Accinelli, C., Abbas, H.K., Shier, W.T.: A bioplastic-based seed coating improves seedling growth and reduces production of coated seed dust. J. Crop Improv. 32, 318–330 (2018)
Accinelli, C., Abbas, H.K., Shier, W.T., Vicari, A., Little, N.S., Aloise, M.R., Giacomini, S.: Degradation of microplastic seed film-coating fragments in soil. Chemosphere 226, 645–650 (2019)
Adrian, Y.F., Schneidewind, U., Bradford, S.A., Simunek, J., Fernandez-Steeger, T.M., Azzam, R.: Transport and retention of surfactant-and polymer-stabilized engineered silver nanoparticles in silicate-dominated aquifer material. Environ. Pollut. 236, 195–207 (2018)
Adrian, Y.F., Schneidewind, U., Bradford, S.A., Šimůnek, J., Klumpp, E., Azzam, R.: Transport and retention of engineered silver nanoparticles in carbonate-rich sediments in the presence and absence of soil organic matter. Environ. Pollut. 255, 113–124 (2019)
Alfaro-Núñez, A., Astorga, D., Cáceres-Farías, L., Bastidas, L., Soto Villegas, C., Macay, K.C., Christensen, J.H.: Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci Rep 11, 6424 (2021)
Alimi, O.S., Farner Budarz, J., Hernandez, L.M., Tufenkji, N.: Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 52, 1704–1724 (2018)
Alimi, O.S., Farner, J.M., Tufenkji, N.: Exposure of nanoplastics to freeze-thaw leads to aggregation and reduced transport in model groundwater environments. Water Res. 189, 116533 (2021)
Allen, S., Allen, D., Phoenix, V.R., Le Roux, G., Durántez Jiménez, P., Simonneau, A., Binet, S., Galop, D.: Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 12, 339–344 (2019)
Atugoda, T., Wijesekara, H., Werellagama, D., Jinadasa, K., Bolan, N.S., Vithanage, M.: Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: implications for vector transport in water. Environ. Technol. Innov. 19, 100971 (2020)
Bettas Ardisson, G., Tosin, M., Barbale, M., Degli-Innocenti, F.: Biodegradation of plastics in soil and effects on nitrification activity. A laboratory approach. Front. Microbiol. 5, 710 (2014)
Bläsing, M., Amelung, W.: Plastics in soil: analytical methods and possible sources. Sci. Total. Environ. 612, 422–435 (2018)
Boos, J.P., Gilfedder, B.S., Frei, S.: Tracking microplastics across the streambed interface: using laser-induced-fluorescence to quantitatively analyze microplastic transport in an experimental flume. Water Resour. Res. 57(12), e2021WR031064 (2021)
Borrelle, S.B., Ringma, J., Law, K.L., Monnahan, C.C., Lebreton, L., McGivern, A., Murphy, E., Jambeck, J., Leonard, G.H., Hilleary, M.A.: Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518 (2020)
Bradford, S.A., Kim, H., Shen, C., Sasidharan, S., Shang, J.: Contributions of nanoscale roughness to anomalous colloid retention and stability behavior. Langmuir 33, 10094–10105 (2017)
Brodhagen, M., Goldberger, J.R., Hayes, D.G., Inglis, D.A., Marsh, T.L., Miles, C.: Policy considerations for limiting unintended residual plastic in agricultural soils. Environ. Sci. Policy 69, 81–84 (2017)
Browne, M.A., Crump, P., Niven, S.J., Teuten, E., Tonkin, A., Galloway, T., Thompson, R.: Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 45, 9175–9179 (2011)
Büks, F., Kaupenjohann, M.: Global concentrations of microplastics in soils—a review. SOIL 6, 649–662 (2020)
Caputo, F., Vogel, R., Savage, J., Vella, G., Law, A., Della Camera, G., Hannon, G., Peacock, B., Mehn, D., Ponti, J.: Measuring particle size distribution and mass concentration of nanoplastics and microplastics: addressing some analytical challenges in the sub-micron size range. J. Colloid. Interface. Sci. 588, 401–417 (2021)
Castan, S., Henkel, C., Hüffer, T., Hofmann, T.: Microplastics and nanoplastics barely enhance contaminant mobility in agricultural soils. Commun. Earth Environ. 2(1), 1–9 (2021)
Coors, A., Edwards, M., Lorenz, P., Römbke, J., Schmelz, R.M., Topp, E., Waszak, K., Wilkes, G., Lapen, D.R.: Biosolids applied to agricultural land: influence on structural and functional endpoints of soil fauna on a short-and long-term scale. Sci. Total. Environ. 562, 312–326 (2016)
Corradini, F., Meza, P., Eguiluz, R., Casado, F., Huerta-Lwanga, E., Geissen, V.: Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total. Environ. 671, 411–420 (2019)
Cowger, W., Booth, A.M., Hamilton, B.M., Thaysen, C., Primpke, S., Munno, K., Lusher, A.L., Dehaut, A., Vaz, V.P., Liboiron, M.: Reporting guidelines to increase the reproducibility and comparability of research on microplastics. Appl Spectrosc 74, 1066–1077 (2020)
Cramer, A., Benard, P., Zarebanadkouki, M., Kaestner, A., Carminati, A.: Microplastic induces soil water repellency and limits capillary flow. Vadose zone j. (2022). https://doi.org/10.1002/vzj2.20215
Danso, D., Chow, J., Streit, W.R.: Plastics: environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 85, e1095–19 (2019)
David, J., Steinmetz, Z., Kučerík, J., Schaumann, G.E.: Quantitative analysis of poly(ethylene terephthalate) Microplastics in soil via thermogravimetry-mass spectrometry. Anal. Chem. 90, 8793–8799 (2018)
Dong, S., Xia, J., Sheng, L., Wang, W., Liu, H., Gao, B.: Transport characteristics of fragmental polyethylene glycol terephthalate (PET) microplastics in porous media under various chemical conditions. Chemosphere 276, 130214 (2021b)
Dong, Y., Gao, M., Qiu, W., Song, Z.: Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 211, 111899 (2021a)
Dong, Z., Qiu, Y., Zhang, W., Yang, Z., Wei, L.: Size-dependent transport and retention of micron-sized plastic spheres in natural sand saturated with seawater. Water Res. 143, 518–526 (2018)
Drummond, J.D., Schneidewind, U., Li, A., Hoellein, T.J., Krause, S., Packman, A.I.: Microplastic accumulation in riverbed sediment via hyporheic exchange from headwaters to mainstems. Sci. Adv. 8, eabi9305 (2022)
Dümichen, E., Eisentraut, P., Bannick, C.G., Barthel, A.-K., Senz, R., Braun, U.: Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 174, 572–584 (2017)
Eerkes-Medrano, D., Thompson, R.: Occurrence, fate, and effect of microplastics in freshwater systems. In: Microplastic contamination in aquatic environments, pp. 95–132. Elsevier, Amsterdam (2018)
APE Europe: Plasticulture in Europe (2019). https://apeeurope.eu/statistics/, Accessed 15 June 2022
FAO: Assessment of agricultural plastics and their sustainability—a call for action. FAO, Rome (2021)
Fischer, M., Scholz-Böttcher, B.M.: Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environ. Sci. Technol. 51, 5052–5060 (2017)
Frei, S., Piehl, S., Gilfedder, B.S., Löder, M.G.J., Krutzke, J., Wilhelm, L., Laforsch, C.: Occurence of microplastics in the hyporheic zone of rivers. Sci Rep 9(1), 1–11 (2019)
Galgani, F., Hanke, G., Werner, S., De Vrees, L.: Marine litter within the European marine strategy framework directive. Ices J. Mar. Sci. 70, 1055–1064 (2013)
Gatidou, G., Arvaniti, O.S., Stasinakis, A.S.: Review on the occurrence and fate of microplastics in sewage treatment plants. J. Hazard. Mater. 367, 504–512 (2019)
Gillefalk, M., Massmann, G., Nützmann, G., Hilt, S.: Potential impacts of induced bank filtration on surface water quality: a conceptual framework for future research. Water 10(9), 1240 (2018)
Goeppert, N., Goldscheider, N.: Experimental field evidence for transport of microplastic tracers over large distances in an alluvial aquifer. J. Hazard. Mater. 408, 124844 (2021)
Gregory, M.R.: Plastic “scrubbers” in hand cleansers: a further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 32, 867–871 (1996)
Haider, T.P., Völker, C., Kramm, J., Landfester, K., Wurm, F.R.: Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 58(1), 50–62 (2019)
Hartmann, N.B., Hüffer, T., Thompson, R.C., Hassellöv, M., Verschoor, A., Daugaard, A.E., Rist, S., Karlsson, T., Brennholt, N., Cole, M., Herrling, M.P., Hess, M.C., Ivleva, N.P., Lusher, A.L., Wagner, M.: Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environmental Science & Technology 53, 1039–1047 (2019)
Heinze, W.M., Mitrano, D.M., Lahive, E., Koestel, J., Cornelis, G.: Nanoplastic transport in soil via bioturbation by Lumbricus terrestris. Environ. Sci. Technol. 55, 16423–16433 (2021)
Helmberger, M.S., Tiemann, L.K., Grieshop, M.J.: Towards an ecology of soil microplastics. Funct Ecology 34, 550–560 (2020)
Hoellein, T.J., Shogren, A.J., Tank, J.L., Risteca, P., Kelly, J.J.: Microplastic deposition velocity in streams follows patterns for naturally occurring allochthonous particles. Sci Rep 9, 1–11 (2019)
Hoggan, J.L., Sabatini, D.A., Kibbey, T.C.: Transport and retention of TiO2 and polystyrene nanoparticles during drainage from tall heterogeneous layered columns. J. Contam. Hydrol. 194, 30–35 (2016)
Horton, A.A., Walton, A., Spurgeon, D.J., Lahive, E., Svendsen, C.: Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 586, 127–141 (2017)
Hou, J., Xu, X., Lan, L., Miao, L., Xu, Y., You, G., Liu, Z.: Transport behavior of micro polyethylene particles in saturated quartz sand: impacts of input concentration and physicochemical factors. Environ. Pollut. 263, 114499 (2020)
Huerta Lwanga, E., Mendoza Vega, J., Ku Quej, V., de Los Angeles Chi, J., Sanchez del Cid, L., Chi, C., Escalona Segura, G., Gertsen, H., Salánki, T., van der Ploeg, M.: Field evidence for transfer of plastic debris along a terrestrial food chain. Sci Rep 7, 1–7 (2017)
Hüffer, T., Metzelder, F., Sigmund, G., Slawek, S., Schmidt, T.C., Hofmann, T.: Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total. Environ. 657, 242–247 (2019)
Hurley, R.R., Nizzetto, L.: Fate and occurrence of micro (nano) plastics in soils: knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 1, 6–11 (2018)
Ingraffia, R., Amato, G., Bagarello, V., Carollo, F.G., Giambalvo, D., Iovino, M., Lehmann, A., Rillig, M.C., Frenda, A.S.: Polyester microplastic fibers affect soil physical properties and erosion as a function of soil type. Soil 8(1), 421–435 (2022)
Iyare, P.U., Ouki, S.K., Bond, T.: Microplastics removal in wastewater treatment plants: a critical review. Environ. Sci. Water Res. Technol. 6, 2664–2675 (2020)
Jiang, Y., Yin, X., Xi, X., Guan, D., Sun, H., Wang, N.: Effect of surfactants on the transport of polyethylene and polypropylene microplastics in porous media. Water Res. 196, 117016 (2021)
Johnson, A.C., Ball, H., Cross, R., Horton, A.A., Jurgens, M.D., Read, D.S., Vollertsen, J., Svendsen, C.: Identification and quantification of microplastics in potable water and their sources within water treatment works in England and Wales. Environ. Sci. Technol. 54, 12326–12334 (2020)
Kane, I.A., Clare, M.A.: Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: a review and future directions. Front. Earth Sci. (2019). https://doi.org/10.3389/feart.2019.00080
Käppler, A., Fischer, D., Oberbeckmann, S., Schernewski, G., Labrenz, M., Eichhorn, K.-J., Voit, B.: Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem 408, 8377–8391 (2016)
Katsumi, N., Kusube, T., Nagao, S., Okochi, H.: Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere 267, 129185 (2021)
Keller, A.S., Jimenez-Martinez, J., Mitrano, D.M.: Transport of nano-and microplastic through unsaturated porous media from sewage sludge application. Environ. Sci. Technol. 54, 911–920 (2019)
Kernchen, S., Löder, M.G.J., Fischer, F., Fischer, D., Moses, S.R., Georgi, C., Nölscher, A.C., Held, A., Laforsch, C.: Airborne microplastic concentrations and deposition across the Weser River catchment. Sci. Total. Environ. 818, 151812 (2022)
Khant, N.A., Kim, H.: Review of current issues and management strategies of microplastics in groundwater environments. Water 14, 1020 (2022)
Koelmans, A.A., Nor, N.H.M., Hermsen, E., Kooi, M., Mintenig, S.M., De France, J.: Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 155, 410–422 (2019)
Kole, P.J., Löhr, A.J., Van Belleghem, F.G.A.J., Ragas, A.M.J.: Wear and tear of tyres: a stealthy source of microplastics in the environment. IJERPH 14, 1265 (2017)
Krause, S., Baranov, V., Nel, H.A., Drummond, J.D., Kukkola, A., Hoellein, T., Smith, G.H.S., Lewandowski, J., Bonet, B., Packman, A.I.: Gathering at the top? Environmental controls of microplastic uptake and biomagnification in freshwater food webs. Environ. Pollut. 268, 115750 (2021)
Kukkola, A., Krause, S., Lynch, I., Smith, G.H.S., Nel, H.: Nano and microplastic interactions with freshwater biota-Current knowledge, challenges and future solutions. Environ Int 152, 106504 (2021)
Kumar, M., Xiong, X., He, M., Tsang, D.C.W., Gupta, J., Khan, E., Harrad, S., Hou, D., Ok, Y.S., Bolan, N.S.: Microplastics as pollutants in agricultural soils. Environ. Pollut. 265, 114980 (2020)
Kwak, J.I., Liu, H., Wang, D., Lee, Y.H., Lee, J.-S., An, Y.-J.: Critical review of environmental impacts of microfibers in different environmental matrices. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 251, 109196 (2022)
Li, J., Liu, H., Paul Chen, J.: Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 137, 362–374 (2018a)
Li, J., Zhang, K., Zhang, H.: Adsorption of antibiotics on microplastics. Environ. Pollut. 237, 460–467 (2018b)
Li, M., He, L., Zhang, M., Liu, X., Tong, M., Kim, H.: Cotransport and deposition of iron oxides with different-sized plastic particles in saturated quartz sand. Environ. Sci. Technol. 53(7), 3547–3557 (2019)
Li, W., Wufuer, R., Duo, J., Wang, S., Luo, Y., Zhang, D., Pan, X.: Microplastics in agricultural soils: extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total. Environ. 749, 141420 (2020)
Liang, Y., Luo, Y., Shen, C., Bradford, S.A.: Micro-and nanoplastics retention in porous media exhibits different dependence on grain surface roughness and clay coating with particle size. Water Res. (2022). https://doi.org/10.1016/j.watres.2022.118717
Liu, E.K., He, W.Q., Yan, C.R.: ‘White revolution’ to ‘white pollution’—agricultural plastic film mulch in China. Environ. Res. Lett. 9, 91001 (2014)
Lozano, Y.M., Lehnert, T., Linck, L.T., Lehmann, A., Rillig, M.C.: Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 12, 169 (2021)
Maes, T., Jessop, R., Wellner, N., Haupt, K., Mayes, A.G.: A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci Rep 7, 44501 (2017)
Möller, J.N., Löder, M.G., Laforsch, C.: Finding microplastics in soils: a review of analytical methods. Environ. Sci. Technol. 54, 2078–2090 (2020)
Mu, H., Wang, Y., Zhang, H., Guo, F., Li, A., Zhang, S., Liu, S., Liu, T.: High abundance of microplastics in groundwater in Jiaodong Peninsula, China. Sci. Total. Environ. (2022). https://doi.org/10.1016/j.scitotenv.2022.156318
Nel, H.A., Smith, G.H.S., Harmer, R., Sykes, R., Schneidewind, U., Lynch, I., Krause, S.: Citizen science reveals microplastic hotspots within tidal estuaries and the remote Scilly Islands, United Kingdom. Mar. Pollut. Bull. 161, 111776 (2020)
Nel, H.A., Chetwynd, A.J., Kelleher, L., Lynch, I., Mansfield, I., Margenat, H., Onoja, S., Oppenheimer, P.G., Smith, G.H.S., Krause, S.: Detection limits are central to improve reporting standards when using Nile red for microplastic quantification. Chemosphere 263, 127953 (2021)
Nelson, T.F., Remke, S.C., Kohler, H.-P.E., McNeill, K., Sander, M.: Quantification of synthetic polyesters from biodegradable mulch films in soils. Environ. Sci. Technol. 54, 266–275 (2019)
O’Connor, D., Pan, S., Shen, Z., Song, Y., Jin, Y., Wu, W.-M., Hou, D.: Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 249, 527–534 (2019)
OECD: Global plastics outlook: economic drivers, environmental impacts and policy options. OECD Publishing, Paris (2022)
Oldfield, L.E., Roy, J.W., Robinson, C.E.: Investigating the use of the artificial sweetener acesulfame to evaluate septic system inputs and their nutrient loads to streams at the watershed scale. J. Hydrol. Reg. Stud. 587, 124918 (2020)
Palsikowski, P.A., Roberto, M.M., Sommaggio, L.R., Souza, P., Morales, A.R., Marin-Morales, M.A.: Ecotoxicity evaluation of the biodegradable polymers PLA, PBAT and its blends using Allium cepa as test organism. J. Polym. Environ. 26(3), 938–945 (2018)
Panno, S.V., Kelly, W.R., Scott, J., Zheng, W., McNeish, R.E., Holm, N., Hoellein, T.J., Baranski, E.L.: Microplastic contamination in karst groundwater systems. Groundwater 57, 189–196 (2019)
Philipp, M., Bucheli, T.D., Kaegi, R.: The use of surrogate standards as a QA/QC tool for routine analysis of microplastics in sewage sludge. Sci. Total. Environ. 835, 155485 (2022)
Piehl, S., Leibner, A., Löder, M.G., Dris, R., Bogner, C., Laforsch, C.: Identification and quantification of macro-and microplastics on an agricultural farmland. Sci Rep 8, 1–9 (2018)
PlasticEurope: Plastics—the facts 2019. An analysis of European plastics production, demand and waste data (2019). https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019. Accessed 15 June 2022
Prata, J.C., da Costa, J.P., Lopes, I., Andrady, A.L., Duarte, A.C., Rocha-Santos, T.: A one health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total. Environ. 777, 146094 (2021)
Puglisi, E., Romaniello, F., Galletti, S., Boccaleri, E., Frache, A., Cocconcelli, P.S.: Selective bacterial colonization processes on polyethylene waste samples in an abandoned landfill site. Sci Rep 9, 1–13 (2019)
Pulido-Reyes, G., Mitrano, D.M., Kägi, R., Gunten, U.V.: The effect of drinking water ozonation on different types of submicron plastic particles. In: International conference on microplastic pollution in the mediterranean sea, pp. 152–157. Springer, Cham (2019)
Pulido-Reyes, G., Magherini, L., Bianco, C., Sethi, R., von Gunten, U., Kaegi, R., Mitrano, D.M.: Nanoplastics removal during drinking water treatment: laboratory-and pilot-scale experiments and modeling. J. Hazard. Mater. 436, 129011 (2022)
Puoci, F., Iemma, F., Spizzirri, U.G., Cirillo, G., Curcio, M., Picci, N.: Polymer in agriculture: a review. Am. J. Agric. Biol. Sci. 3, 299–314 (2008)
Qi, R., Jones, D.L., Li, Z., Liu, Q., Yan, C.: Behavior of microplastics and plastic film residues in the soil environment: a critical review. Sci. Total. Environ. 703, 134722 (2020)
Qian, H., Zhang, M., Liu, G., Lu, T., Qu, Q., Du, B., Pan, X.: Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut 229, 1–11 (2018)
Ramos, L., Berenstein, G., Hughes, E.A., Zalts, A., Montserrat, J.M.: Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total. Environ. 523, 74–81 (2015)
Re, V.: Shedding light on the invisible: addressing the potential for groundwater contamination by plastic microfibers. Hydrogeol J 27, 2719–2727 (2019)
Rillig, M.C., Ziersch, L., Hempel, S.: Microplastic transport in soil by earthworms. Sci Rep 7, 1–6 (2017)
Rochman, C.M., Brookson, C., Bikker, J., Djuric, N., Earn, A., Bucci, K., Athey, S., Huntington, A., McIlwraith, H., Munno, K.: Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 38, 703–711 (2019)
Rodríguez-Seijo, A., Pereira, R.: Microplastics in agricultural soils: Are they a real environmental hazard? In: Bioremediation of agricultural soils, pp. 45–60. CRC Press, Boca Raton (2019)
Ron, C.A., VanNess, K., Rasmuson, A., Johnson, W.P.: How nanoscale surface heterogeneity impacts transport of nano- to micro-particles on surfaces under unfavorable attachment conditions. Environ. Sci. Nano 6, 1921–1931 (2019)
Sajjad, M., Huang, Q., Khan, S., Khan, M.A., Yin, L., Junfeng, W., Faqin, L., Qingqing, W., Guo, G.: Microplastics in the soil environment: a critical review. Environ. Technol. Innov. (2022). https://doi.org/10.1016/j.eti.2022.102408
Samandra, S., Johnston, J.M., Jaeger, J.E., Symons, B., Xie, S., Currell, M., Ellis, A.V., Clarke, B.O.: Microplastic contamination of an unconfined groundwater aquifer in Victoria. Aust. Sci. Total. Environ. 802, 149727 (2022)
Scheurer, M., Bigalke, M.: Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 52, 3591–3598 (2018)
Schönenberger, U.T., Beck, B., Dax, A., Vogler, B., Stamm, C.: Pesticide concentrations in agricultural storm drainage inlets of a small Swiss catchment. Environ. Sci. Pollut. Res. (2022). https://doi.org/10.1007/s11356-022-18933-5
Schrank, I., Möller, J.N., Imhof, H.K., Hauenstein, O., Zielke, F., Agarwal, S., Löder, M.G., Greiner, A., Laforsch, C.: Microplastic sample purification methods-Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total. Environ. 833, 154824 (2022)
Schwaferts, C., Sogne, V., Welz, R., Meier, F., Klein, T., Niessner, R., Elsner, M., Ivleva, N.P.: Nanoplastic analysis by online coupling of Raman microscopy and field-flow fractionation enabled by optical tweezers. Anal. Chem. 92, 5813–5820 (2020)
Schymanski, D., Goldbeck, C., Humpf, H.-U., Fürst, P.: Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. 129, 154–162 (2018)
Seidensticker, S., Grathwohl, P., Lamprecht, J., Zarfl, C.: A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ Sci Eur 30, 1–12 (2018)
Serrano-Ruiz, H., Martin-Closas, L., Pelacho, A.M.: Biodegradable plastic mulches: impact on the agricultural biotic environment. Sci. Total. Environ. 750, 141228 (2021)
Shi, J., Dong, Y., Shi, Y., Yin, T., He, W., An, T., Tang, Y., Hou, X., Chong, S., Chen, D.: Groundwater antibiotics and microplastics in a drinking-water source area, northern China: occurrence, spatial distribution, risk assessment, and correlation. Environ. Res. 210, 112855 (2022)
de Souza Machado, A.A., Kloas, W., Zarfl, C., Hempel, S., Rillig, M.C.: Microplastics as an emerging threat to terrestrial ecosystems. Glob Change Biol 24, 1405–1416 (2018)
Steinmetz, Z., Wollmann, C., Schaefer, M., Buchmann, C., David, J., Tröger, J., Muñoz, K., Frör, O., Schaumann, G.E.: Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total. Environ. 550, 690–705 (2016)
Tamang, A., Roy, J.W., Boreux, M.P., Robinson, C.E.: Variation in septic system effluent inputs to tributaries in multiple subwatersheds and approaches to distinguish contributing pathways and areas. Sci. Total. Environ. 807, 151054 (2022)
The Royal Society: Microplastics in freshwater and soil (2019). https://royalsociety.org/topics-policy/projects/microplastics-in-freshwater-and-soils/, Accessed 15 June 2022
Tian, L., Jinjin, C., Ji, R., Ma, Y., Yu, X.: Microplastics in agricultural soils: sources, effects, and their fate. Curr. Opin. Environ. Sci. Health 25, 100311 (2022)
Tong, M., Li, T., Li, M., He, L., Ma, Z.: Cotransport and deposition of biochar with different sized-plastic particles in saturated porous media. Sci. Total. Environ. 713, 136387 (2020)
Torkzaban, S., Bradford, S.A., van Genuchten, M.T., Walker, S.L.: Colloid transport in unsaturated porous media: the role of water content and ionic strength on particle straining. J. Contam. Hydrol. 96, 113–127 (2008)
Viaroli, S., Lancia, M., Re, V.: Microplastics contamination of groundwater: current evidence and future perspectives. A review. Sci. Total. Environ. (2022). https://doi.org/10.1016/j.scitotenv.2022.153851
Waldschläger, K., Lechthaler, S., Stauch, G., Schüttrumpf, H.: The way of microplastic through the environment—Application of the source-pathway-receptor model. Sci. Total. Environ. 713, 136584 (2020)
Wang, J., Liu, X., Li, Y., Powell, T., Wang, X., Wang, G., Zhang, P.: Microplastics as contaminants in the soil environment: a mini-review. Sci. Total. Environ. 691, 848–857 (2019)
Wang, W., Ge, J., Yu, X., Li, H.: Environmental fate and impacts of microplastics in soil ecosystems: progress and perspective. Sci. Total. Environ. 708, 134841 (2020)
Wanner, P.: Plastic in agricultural soils—a global risk for groundwater systems and drinking water supplies?—a review. Chemosphere 264, 128453 (2021)
Weithmann, N., Möller, J.N., Löder, M.G., Piehl, S., Laforsch, C., Freitag, R.: Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 4, eaap8060 (2018)
Wu, X., Lyu, X., Li, Z., Gao, B., Zeng, X., Wu, J., Sun, Y.: Transport of polystyrene nanoplastics in natural soils: effect of soil properties, ionic strength and cation type. Sci. Total. Environ. 707, 136065 (2020)
Xu, J.-L., Thomas, K.V., Luo, Z., Gowen, A.A.: FTIR and Raman imaging for microplastics analysis: state of the art, challenges and prospects. Trac Trends Anal. Chem. 119, 115629 (2019)
Yan, X., Yang, X., Tang, Z., Fu, J., Chen, F., Zhao, Y., Ruan, L., Yang, Y.: Downward transport of naturally-aged light microplastics in natural loamy sand and the implication to the dissemination of antibiotic resistance genes. Environ. Pollut. 262, 114270 (2020)
Yang, L., Zhang, Y., Kang, S., Wang, Z., Wu, C.: Microplastics in soil: a review on methods, occurrence, sources, and potential risk. Sci. Total. Environ. 780, 146546 (2021)
Zhang, G., Zhang, F., Li, X.: Effects of polyester microfibers on soil physical properties: perception from a field and a pot experiment. Sci. Total. Environ. 670, 1–7 (2019)
Zubris, K.A.V., Richards, B.K.: Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 138, 201–211 (2005)
Acknowledgements
C. M. acknowledges the financial support of internal Eawag Discretionary Funding. U.S. would like to acknowledge funding from the German Research Foundation (DFG—grant number 403826296). S. K. would like to acknowledge funding from the Royal Society (INF\R2\212060) and Leverhulme Trust (RPG-2017-377 and RPG-2021-030).
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Moeck, G. Davies, S. Krause and U. Schneidewind declare that they have no competing interests. Opinions presented in this work are those of the authors and not necessarily of the funding agencies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moeck, C., Davies, G., Krause, S. et al. Microplastics and nanoplastics in agriculture—A potential source of soil and groundwater contamination?. Grundwasser - Zeitschrift der Fachsektion Hydrogeologie 28, 23–35 (2023). https://doi.org/10.1007/s00767-022-00533-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00767-022-00533-2