Abstract
A novel, green, and cost-effective thin-layer chromatography (TLC)‒spectrodensitometric method was designed and validated for the simultaneous determination of a five-component mixture. The analyzed mixture is composed of three active ingredients: propyphenazone (PRO), caffeine (CAF), and ergotamine tartrate (ERG), along with two official impurities which are PRO impurity: phenazone (PHN) and CAF impurity: theophylline (THEO). The suggested method was used for the quantitation of the three coformulated active ingredients in their marketed tablet and in human plasma. The studied compounds were separated on TLC silica gel 60F254 plates using a mobile phase consisting of methanol–ethyl acetate–glacial acetic acid (1:9:0.1, V/V) with diprophylline (DPP) as internal standard. Densitometric scanning was carried out at 210.0 nm. Method validation was assessed according to the International Council for Harmonisation (ICH) guidelines. The greenness profile for the proposed method was evaluated using the National Environmental Method Index (NEMI), analytical eco-scale, and Green Analytical Procedure Index (GAPI) tools. The proposed method offers the advantages of being simple, rapid, economic, and ecofriendly. It is a successful choice for the routine analysis of the studied drugs in pharmaceutical and biological samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thin-layer chromatography (TLC) is a well-established separation analytical methodology that has enjoyed wide application in the analytical field owing to its simplicity, available equipment, automation, and low cost. TLC is satisfactorily applied for the analysis of biological matrices as it sets aside the clean-up and sample preparation steps [1]. Recently, the combination of TLC and smartphone applications has been implemented in the quantitative analysis of different compounds [2, 3]. Inspired by these benefits, the primary aim of this work is to develop an accurate, precise, and sensitive method for the analysis of the studied compounds: propyphenazone (PRO), caffeine (CAF), and ergotamine tartrate (ERG), along with PRO impurity: phenazone (PHN) and CAF impurity: theophylline (THEO). Moreover, the method was employed for the determination of the active ingredients in their pharmaceutical formulation and in spiked human plasma.
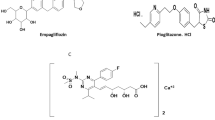
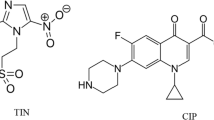
PRO, shown in Fig. 1A [4], is one of the pyrazolone derivatives having both analgesic and antipyretic properties [5]. CAF, shown in Fig. 1B [4], is a central nervous system stimulant. It is commonly added to analgesic preparations to boost analgesia [5]. ERG, shown in Fig. 1C [4], is an alkaloid derivatized from ergot. It has a vasoconstrictor and powerful oxytocic effects on uterus. It is used in migraine conditions that cannot be treated with nonopioid analgesics [5]. According to the British Pharmacopoeia (BP) 2019, PHN, shown in Fig. 1D, is named as PRO-related impurity A. It is a nonsteroidal antiinflammatory drug that has been given orally as an analgesic and used topically as ear drops in acute otitis [5]. THEO, shown in Fig. 1E, is stated as CAF official impurity A [4]. Its pharmacological actions include relaxation of bronchial smooth muscle, relieving bronchospasm, and stimulation of respiration. It also causes diuresis and may have an antiinflammatory effect [5].
Cleamine A1.0® tablets, which contain PRO, CAF, and ERG, are used as analgesics and vasoconstrictors for the relief of vascular headache, migraine, and tension headache.
A few TLC techniques for determining PRO and CAF in pharmaceutical preparations have been reported [6,7,8]. Also, TLC methods have been developed for the simultaneous determination of CAF, ERG, and other active ingredients in their combined tablets and in blood samples [9,10,11]. The literature at hand indicates that no TLC‒spectrodensitometric method has been published, neither for the simultaneous detection of PRO, CAF, and ERG nor in the presence of PRO and CAF impurities.
Consequently, our present research is innovative with respect to its ability to estimate the studied active ingredients along with some of their probable impurities. Additionally, the devised method combined the protein precipitation technique with TLC as a sample preparation step for the identification of the investigated chemicals in human plasma. The method was validated in accordance with the International Council for Harmonisation (ICH) criteria [12].
2 Experimental
2.1 Instruments and software
The separation was performed on 20 cm × 20 cm TLC plates precoated with silica gel 60F254, 0.25 mm thickness (Merck, Darmstadt, Germany). The samples were applied on the plates using a CAMAG Linomat 5 autosampler with a CAMAG microsyringe (100 µL) (CAMAG, Muttenz, Switzerland). A CAMAG TLC Scanner model 3S/N 1302139 with winCATS software was used for densitometric quantification with an ultraviolet (UV) lamp (Vilber Lourmat, Marne-la-Vallée, France) housed in an air-ventilated cabinet. We used a vortex mixer, model no. VM-300 (Gemmy Industrial Corporationm, Taipei, Taiwan).
2.2 Materials and reagents
2.2.1 Pure samples
Pure sample of PRO was supplied by the Egyptian International Pharmaceutical Industries Company (EIPICO; Tenth of Ramadan City, Egypt). The official nonaqueous potentiometric titration method of BP [4] was used to evaluate its purity, yielding a value of 101.72 ± 1.610.
EIPICO provided a pure sample of CAF. Using the official BP method [4], its purity was assessed and found to be 101.67 ± 1.612.
EIPICO provided a pure sample of ERG. Its purity was determined to be 100.49 ± 1.372 according to the BP official method [4].
Pure sample of PHN, “PRO official impurity A” was provided by EIPICO. Its purity was checked according to the BP official redox back-titration method and found to be 101.10 ± 1.256 [4].
Pure sample of THEO, “CAF official impurity A” was provided by EIPICO. According to the official United States Pharmacopeia high-performance liquid chromatography (USP HPLC) procedure [13], its purity was tested and determined to be 99.66 ± 1.955.
Pure sample of diprophylline (DPP) was provided by Amoun Pharmaceutical Company (Obour City, El Qalyubia, Egypt).
2.2.2 Pharmaceutical dosage form
Cleamine A1.0® tablets (batch no. AD3001) were manufactured by Nichi-Iko Pharmaceutical Company (Toyama, Japan). It is stated that every tablet includes 300.00 mg of PRO, 50.00 mg of CAF, and 1.00 mg of ERG. The tablets were purchased from the Family Pharmacy Global website (www.mimaki-family-japan.com) and delivered through Express Mail Service (EMS).
2.2.3 Chemicals and reagents
Analytical-grade chemicals and reagents were used throughout the research: methanol, HPLC grade (Fisher Scientific, Loughborough, UK); ethyl acetate, HPLC grade (Sigma-Aldrich, Poznan, Poland); and glacial acetic acid, analytical grade (El Nasr Pharmaceutical Chemical Company, Cairo, Egypt).
2.2.4 Human plasma
Human plasma samples were obtained from the Holding Company for Biological Products and Vaccines (VACSERA, Giza, Egypt).
2.3 Stock standard solutions
Stock standard solution of each of PRO, CAF, ERG, PHN, and THEO (1.00 mg/mL) was prepared through exact weighing of 0.05 g of each drug and carefully pouring into five different 50-mL volumetric flasks. After adding about 25 mL of methanol and sonicating for a few minutes, the volume was completed to the specified level using the same solvent. ERG stock solution was shielded from light because it darkens and disintegrates when exposed to light, heat, and air [14].
2.4 Procedure
2.4.1 Chromatographic conditions
Silica gel (60F254) precoated 20 cm × 20 cm TLC plates were used for the analysis. A CAMAG Linomat 5 autosampler, equipped with a Hamilton microsyringe (100 µL), was used to apply the five compounds on the TLC plates. The band width of the spots was 6 mm. They were 10 mm apart from the plate’s bottom border and side edges, and spaced by 9.4 mm from each other. Development was carried out in a chromatographic tank saturated with the developing system methanol‒ethyl acetate‒glacial acetic acid (1:9:0.1, V/V) in a linear ascending manner. The mobile phase was ascended over a distance of 8.5 cm, then the plate was left to air-dry at ambient temperature followed by scanning at 210.0 nm to obtain the corresponding peak areas.
2.4.2 Method validation
The proposed method was validated in accordance to ICH guidelines (2005) through assessing linearity, range, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), and robustness.
2.4.2.1 Linearity and range
Stock standard solution (1.00 mg/mL) of each drug was used. Aliquots of PRO (0.1‒12.0 µL), CAF and ERG (0.1‒10.0 µL), and PHN and THEO (0.1‒1.0 µL) were spotted on TLC plates as mentioned in Sect. 2.4.1. The scan profiles for PRO, CAF, ERG, PHN, and THEO were obtained. A second-order polynomial curve relating the average peak area to the corresponding concentration was constructed for each analyte. The corresponding regression equations and correlation coefficients were computed.
2.4.2.2 Accuracy
The accuracy of the results was verified by applying the proposed method for the determination of various samples of PRO, CAF, ERG, PHN, and THEO. The concentrations were acquired via the corresponding regression equations, and the % recoveries were calculated.
2.4.2.3 Precision
-
(a)
Repeatability
Three concentrations of PRO, CAF, and ERG stock standard solutions (4.00, 5.00, and 8.00 µg/band) and three concentrations of PHN and THEO stock standard solutions (0.50, 0.70, and 0.80 µg/band) were analyzed three times each on the same day, using the procedure previously stated in Sect. 2.4.2.1. The relative standard deviation (%RSD) for each of the five compounds was calculated.
-
(b)
Intermediate precision
The same samples used to assess repeatability were analyzed on three different days using the procedure mentioned in Sect. 2.4.2.1. The %RSD for each of the studied compounds was calculated.
2.4.2.4 LOD and LOQ
The minimum concentration at which the two impurities PHN and THEO could be detected (LOD) and the minimum concentration at which they could be determined accurately and precisely (LOQ) were estimated using the signal-to-noise ratio approach.
2.4.2.5 Robustness
The robustness of the proposed method was evaluated upon changing various parameters in the developed method. These parameters include the developing system ratio (±1%), saturation time (±10 min), and time elapsed from chromatographic separation to scanning (±10 min).
2.4.3 Application of the proposed TLC‒spectrodensitometric method for the determination of PRO, CAF, and ERG in Cleamine A1.0® tablets
Determination of PRO and CAF: Ten Cleamine A1.0® tablets were weighed, ground, and thoroughly blended. A quantity from the powder equal to one tablet (containing 300.00 mg PRO, 50.00 mg CAF, and 1.00 mg ERG) was precisely weighed and transferred into a 100-mL beaker. The powder was sonicated for half an hour in about 50 mL of methanol and filtered into a 100-mL volumetric flask. Thirty milliliters of methanol were used to wash the residue for three times (3 × 10). The volume was then completed to the mark with the same solvent. Two microliters of the filtrate (containing 6.00 µg PRO and 1.00 µg CAF) was withdrawn by using the CAMAG microsyringe and spotted on a TLC plate as mentioned in Sect. 2.4.1 for the determination of both active ingredients in Cleamine A1.0® tablets. The validity of the proposed method was further assessed by applying the standard addition technique.
Determination of ERG: An amount of the Cleamine A1.0® tablet powder equivalent to one tablet (containing 1.00 mg ERG) was accurately weighed and transferred into a 50-mL beaker. It was sonicated for 30 min in about 20 mL of methanol and filtered into a 50-mL volumetric flask. The residue was washed twice each using 10 mL methanol, and the volume was completed to the mark with methanol. Twenty microliters from the filtered solution (containing 0.40 µg ERG) was withdrawn by using the CAMAG microsyringe and spotted on a TLC plate as mentioned in Sect. 2.4.1 for the determination of ERG in Cleamine A1.0® tablets. The validity of the proposed method was further assessed by applying the standard addition technique.
2.4.4 Biological application of the proposed TLC‒spectrodensitometric method on spiked human plasma
2.4.4.1 Stock standard solution of PRO, CAF, and ERG (4.00 mg/mL)
A stock standard solution of PRO, CAF, and ERG each (4.00 mg/mL) was prepared separately in methanol by accurately weighing 0.20 g of the three pure powders into a 50-mL volumetric flask. About 25 mL of methanol was added, sonicated for few minutes, and the volume was then completed to the mark with methanol and protected from light.
2.4.4.2 Stock standard solution of the internal standard DPP (1.00 mg/mL)
A stock standard solution of the internal standard (IS): DPP (1.00 mg/mL) was prepared in methanol by accurately weighing 0.05 g of the pure powder into a 50-mL volumetric flask. About 25 mL of methanol was added, sonicated for few minutes, and the volume was then completed to the mark with methanol.
2.4.4.3 Working standard solutions (calibrators) and quality control samples of PRO, CAF, and ERG
Calibrators and quality control (QC) samples were prepared by appropriate dilutions from stock standard solution of PRO, CAF, and ERG (4.00 mg/mL) into a series of 10-mL volumetric flasks. These dilutions resulted in six working solutions (200.00 µg/mL, 300.00 µg/mL, 400.00 µg/mL, 500.00 µg/mL, 1000.00 µg/mL, and 2000.00 µg/mL). These solutions were used to construct calibration curves for each drug in plasma. Moreover, three QC samples working solutions were prepared in a similar manner at low quality control (LQC, 600.00 µg/mL), mid quality control (MQC, 800.00 µg/mL), and high quality control (HQC, 1600.00 µg/mL).
2.4.4.4 Working standard solution of the internal standard DPP (400.00 µg/mL)
Four milliliters of the stock standard solution of DPP (1.00 mg/mL) was accurately transferred into a 10-mL volumetric flask. The volume was completed to the mark with methanol, and the obtained solution was mixed well.
2.4.4.5 Preparation of calibration and quality control samples in human plasma
A fixed volume (450.0 μL) of drug-free human plasma sample was accurately withdrawn and put into nine centrifugation tubes, and spiked with exactly 50.0 μL of the prepared six working standard solutions on the day of analysis. Similar steps were employed to prepare three QC samples in human plasma. The nine samples of spiked human plasma were held at 20 °C in tightly closed, light-resistant containers before analysis. All plasma samples were immediately thawed at room temperature before being analyzed.
2.4.4.6 Sample preparation for human plasma extraction
A fixed volume (50.0 μL) of internal standard DPP working standard solution (400.00 µg/mL) was added to the 500.0 μL of spiked human plasma samples described above. They were vortex-blended for 1 min. The precipitation process was done by adding 1.0 mL of acetonitrile, vortexing for 1 min, and then centrifuging at 4000 rpm for 10 min. A certain volume (10.0 µL) of the clear upper organic phase from each centrifugation tube was spotted on the TLC plate. The general process was then followed as detailed in Sect. 2.4.1.
3 Results and discussion
In this work, an ecofriendly, sensitive, and precise TLC‒spectrodensitometric method was developed for the determination of the five studied compounds PRO, CAF, ERG, PHN, and THEO. The proposed method was successfully applied on Cleamine A1.0® tablets and on spiked human plasma using the internal standard, DPP.
3.1 Optimization of chromatographic conditions
It was important to investigate the impact of several experimental conditions in order to optimize the chromatographic separation. The studied conditions include:
-
(a)
Effect of mobile phase type and composition.
There is a worldwide attempt to accomplish green analytical chemistry (GAC), which assures safety and healthiness of the environment [15]. In accordance, several trials using various ecofriendly mobile phases of various compositions and ratios were held. The commonly employed dangerous solvents such as tetrahydrofuran, chloroform, and toluene were omitted in all trials. The system of methanol‒ethyl acetate‒glacial acetic acid (1:9:0.1, V/V) was shown to be the most effective for separation. This selected mobile phase allowed the separation and quantification of the five compounds where the separated bands were well shaped with satisfactory retardation factor (RF). The obtained two-dimensional (2D) and three-dimensional (3D) scanning profiles for the separated compounds PRO, CAF, ERG, PHN, and THEO are shown in Fig. 2A and B, respectively. It is worth noting that the impurities PHEN and THEO could be determined accurately up to 10% of CAF concentration, as above this limit PHEN and THEO peaks would interfere with CAF peak.
A 2D TLC chromatogram of a resolved mixture of 12 µg/band PRO, 10 µg/band CAF, 0.1 µg/band of ERG, propyphenazone impurity PHN, and caffeine impurity THEO. B 3D scanning profile of the TLC chromatogram of propyphenazone (0.10‒12.00 µg/band), caffeine (0.10‒10.00 µg/band), ergotamine tartrate (0.10‒10.00 µg/band), and 0.10‒1.00 µg/band of phenazone and theophylline
-
(b)
Band dimensions.
Ordinary diffusion may result in slight spreading of the applied bands. Also, narrow band width may lead to overloading of silica, resulting in tailing and mistaken results. Therefore, choosing the band width and the spaces between bands is crucial to avoid interference between adjacent bands and inaccurate results. The ideal band width was chosen to be 6 mm with a 9.4 mm interspace between bands.
-
(c)
Scanning wavelength.
On the basis of the UV absorption spectra of the five compounds shown in Fig. 3, the wavelength of 210.0 nm was chosen for their determination. The five compounds showed great sensitivity at the selected wavelength, at which sharp and symmetric peaks were obtained with a single plate scan.
3.2 System suitability
System suitability parameters were assessed in accordance with USP [16]. The obtained results were satisfactory, as demonstrated in Table 1. The resolution values (Rs) for the bands were above “one.” The selectivity factor (α) was also greater than “one,” which assures adequate separation. The asymmetry factors were 0.88, 1.00, 1.10, 1.19, and 0.83 for PRO, CAF, ERG, PHN, and THEO, respectively, revealing satisfactory peak elution without tailing. The RF values were 0.84 ± 0.10, 0.56 ± 0.10, 0.18 ± 0.10, 0.41 ± 0.10, and 0.64 ± 0.10 for PRO, CAF, ERG, PHN, and THEO, respectively.
3.3 Validation of the proposed TLC‒spectrodensitometric method
The validity of the proposed method was assessed according to ICH 2005 guidelines, and acceptable results were obtained (Table 2).
3.3.1 Linearity and range
Interpretation of the regression parameters was carried out for the five studied compounds PRO, CAF, ERG, PHN, and THEO in their pure standards using the developed method. The estimated parameters are illustrated in Table 2.
3.3.2 Accuracy
The accuracy of the method was assessed for different samples of the studied compounds in their pure forms. The calculated mean % recovery verified that the suggested procedure is accurate (Table 2).
3.3.3 Precision
Acceptable %RSD values were obtained (Table 2) for the intraday and interday analyzed samples, indicating that the implemented method is precise.
3.3.4 LOD and LOQ
The low estimated LOD and LOQ concentrations, listed in Table 2, for the two impurities PHN and THEO by signal-to-noise ratio reveal the suggested method’s great sensitivity.
3.3.5 Robustness
The calculated %RSD of the tailing factor (T) of the five analyzed components upon changing the developing system ratio (±1%), saturation time (±10 min), and time from separation to scanning (±10 min) guarantees that the proposed method is robust (Table 2).
3.4 Quantitative determination of PRO, CAF, and ERG in Cleamine A1.0® tablets using the proposed TLC‒spectrodensitometric method
The estimated percentage recoveries, shown in Table 3, assure the suitability of the proposed method for the routine analysis of PRO, CAF, and ERG in their combined tablets without interference from tablet excipients. Standard addition technique was accomplished through adding appropriate amounts of standards PRO, CAF, and ERG to appropriate weighed powdered Cleamine A1.0® tablets, and the solid mixtures were extracted with methanol and analyzed as shown in Table 3.
3.5 Determination of PRO, CAF, and ERG in spiked human plasma using the proposed TLC‒spectrodensitometric method
Sample preparation in bioanalytical methodologies aids augmentations of sensitivity and selectivity. The simplest and low-priced sample preparation approach is protein precipitation [1]. Thus, in this study, the protein precipitation approach was chosen for sample preparation using acetonitrile. The prepared plasma samples were then analyzed for their PRO, CAF, and ERG content using the proposed TLC‒spectrodensitometric method. Regarding the internal standard choice, several compounds (amlodipine, ephedrine, and phenobarbital) with similar physicochemical characteristics, viz. log partition coefficient (log P) and pKa , to the active ingredients in Cleamine A1.0® tablet were tested as internal standards. However, DPP was selected as internal standard in our study as it showed the best resolution from the studied compounds without interference from plasma components, as illustrated in Fig. 4.
Table 4 summarizes the regression equation parameters, accuracy, and precision of the proposed TLC‒spectrodensitometric method for the determination of PRO, CAF, and ERG in spiked human plasma. The obtained values were within the accepted ranges as specified in FDA Bioanalytical Method Validation Guidance for Industry [17].
3.6 Statistical comparison
The results obtained by applying the proposed method for the analysis of the five compounds were statistically compared with those obtained by the official methods for their determination. The obtained calculated t and F-values were found to be less than the corresponding theoretical ones. This proves that there is no significant difference between the proposed method and the official ones regarding both accuracy and precision (Table 5).
3.7 Greenness assessment of the proposed method
Three different approaches were employed to assure that the proposed method is safe to the environment, first by means of the qualitative National Environmental Methods Index (NEMI) [18]. In this tool, analytical procedures are evaluated using a NEMI label, which is a circle divided into four quarters. Each quarter represents a certain aspect of the described analytical methodology. The first quarter is colored green if none of the chemicals used in the method exists in the persistent, bio-accumulative, and toxic chemicals list (“PBT” field). If there are no used chemicals listed under D, F, P, or U hazardous wastes, the second quarter is colored green (“Hazardous” field). If the pH of the examined samples falls in the range of 2–12, the third quarter is colored green (“Corrosive” field). The technique must yield no more than 50 g of waste in order to color the fourth quarter in green (“Waste” field). The second approach used for greenness assessment is the semiquantitative analytical eco-scale method [19]. This approach is based on calculating penalty points for each aspect of the analytical procedure and then subtracting the total penalty points from 100. A method is designated as a perfect green method if it receives a score of 100 on the eco-scale, an excellent green method if it receives a score of > 75, and an acceptable green method if it receives a score of > 50, while the procedure would be considered insufficiently green if the eco-scale result is less than 50. A detailed explanation of how to calculate the penalty points for an analytical procedure is presented by Gałuszka et al. [19]. To provide complete information about the greenness of the proposed analytical method, a new tool called the Green Analytical Procedure Index (GAPI) has been exploited [20]. In GAPI, a specific symbol with five pentagrams can be used to assess the greenness of each stage of an analytical procedure. The five pentagrams are subdivided into 15 stages. The GAPI pictogram uses a color scale, with three levels of evaluation for each stage (from stage 1 to 15). The three colors are green, yellow, and red, indicating low, medium, and high environmental impact, respectively. The GAPI parameters description and pictograms are clarified by Płotka-Wasylka. The proposed method proved to be a satisfactory eco-friendly method according to the NEMI, analytical eco-scale (eco-score = 76), and GAPI (Table 6).
4 Conclusions
A novel, economic, and environmentally friendly TLC‒spectrodensitometric method was designed for the quantitation of a five-component mixture. The mixture consists of three active constituents (PRO, CAF, and ERG) along with two impurities, viz. the PRO impurity PHN and CAF impurity THEO. The method was effectively used to quantify the active components PRO, CAF, and ERG in Cleamine A1.0® tablets as well as in spiked human plasma. The developed approach combined the protein precipitation technique with TLC as a step for cleaning the biological samples. The described approach can be employed as an eco-friendly, simple, and accurate method for the analysis of PRO, CAF, and ERG in quality control and bioanalytical laboratories.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rezk MR, Monir HH, Marzouk HM (2019) Novel determination of a new antiviral combination; sofosbuvir and velpatasvir by high performance thin layer chromatographic method; application to real human samples. Microchem J 146:828–834. https://doi.org/10.1016/j.microc.2019.02.012
Wadie M, Abdel-Moety EM, Rezk MR, Marzouk HM (2023) A novel smartphone HPTLC assaying platform versus traditional densitometric method for simultaneous quantification of alfuzosin and solifenacin in their dosage forms as well as monitoring content uniformity and drug residues on the manufacturing equipment. RSC Adv 13:11642–11651. https://doi.org/10.1039/D3RA01211E
Wadie M, Abdel-Moety EM, Rezk MR, Marzouk HM (2023) Sustainable and smart HPTLC determination of silodosin and solifenacin using a constructed two illumination source chamber with a smartphone camera as a detector: comparative study with conventional densitometric scanner. Sustain Chem Pharm 33:101095. https://doi.org/10.1016/j.scp.2023.101095
British Pharmacopoeia (2019) Vol. II. The Stationery Office, London.
Sweetman SC (2012) Martindale: the complete drug reference, 37th edn. The Pharmaceutical Press, London
Kosmeas N, Clerc J (1989) A quick DC method for simultaneous quantitative determination of paracetamol, caffeine, phenobarbital and propyphenazone in plasma. Pharm Acta Helv 64:2–7
Tomankova H, Vasatova M (1989) Densitometric determination of propyphenazone, paracetamol, guaiacol glycerol ether, caffeine and acetylsalicylic acid in analgesic-antipyretic preparations with thin-layer chromatography. Pharmazie 44:197–198
Hałka-Grysinska A, Slazak P, Zareba G, Markowski W, Klimek-Turek A, Dzido TH (2012) Simultaneous determination of acetaminophen, propyphenazone and caffeine in cefalgin preparation by pressurized planar electrochromatography and high-performance thin-layer chromatography. Anal Methods 4:973–982. https://doi.org/10.1039/C2AY05679H
Amin M, Sepp W (1976) Quantitative thin-layer chromatographic analysis of ergotamine tartrate and caffeine in the nanogram range. J Chromatogr A 118:225–232. https://doi.org/10.1016/S0021-9673(00)81211-X
Aranda M, Morlock G (2007) Simultaneous determination of caffeine, ergotamine, and metamizol in solid pharmaceutical formulation by HPTLC-UV-FLD with mass confirmation by online HPTLC-ESI-MS. J Chromatogr Sci 45:251–255. https://doi.org/10.1093/chromsci/45.5.251
Marzouk HM, Ibrahim EA, Hegazy MA, Saad SS (2021) Greenness profile assessment of selective liquid chromatographic methods for determination of a quaternary anti-migraine combination along with three of their related official impurities. Biomed Chromatogr 35(9):e5132. https://doi.org/10.1002/bmc.5132
International Conference on Harmonization (2005) ICH Harmonized Tripartite Guideline Q2(R1): Validation of Analytical Procedures: Text and Methodology, Geneva
The United States Pharmacopeia (2011) USP 34 edn., NF 29. The United States Pharmacopeial Convention, Rockville, MD
Budavari S (1996) The Merck Index, 12th edn. Merck & Co., Inc, Whitehouse Station
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
The United States Pharmacopeia (2019) USP 42 edn., NF 37. The United States Pharmacopeial Convention, Rockville, .M
Bioanalytical Method Validation Guidance for Industry (2018) Center for Drug Evaluation and Research (CDER), Silver Spring, MD
Keith LH, Gron LU, Young JL (2007) Green analytical methodologies. Chem Rev 107:2695–2708. https://doi.org/10.1021/cr068359e
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.T.R.: Conceptualization, methodology, validation, formal analysis, investigation, writing and editing. N.K.R.: Review and editing, supervision, conceptualization. N.A.E.-R.: Review and editing, supervision, conceptualization. B.A.E.-Z.: Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Human plasma samples were supplied by the Holding Company of Biological Products and Vaccines, VACSERA (Giza, Egypt) with permission. The whole study was approved by and supervised under the Internal Ethics Committee of Faculty of Pharmacy, Cairo University.
Consent for publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ragab, M.T., Ramadan, N.K., El-Ragehy, N.A. et al. Thin layer chromatography‒spectrodensitometric determination of a three-component mixture of propyphenazone, caffeine, ergotamine tartrate, and two of their impurities with application to tablets, spiked human plasma, and green profile assessment. JPC-J Planar Chromat 36, 295–305 (2023). https://doi.org/10.1007/s00764-023-00248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-023-00248-x