Abstract
The importance of the binary mixtures of the novel oral anticoagulants (NOACs): apixaban (APX), edoxaban tosylate (EDX) and rivaroxaban (RIV) with the lipid-lowering statin, rosuvastatin calcium (ROS) is highly emerging to save lives of cardiovascular patients as these combinations are used in prophylaxis from stroke. A high-performance thin-layer chromatography (HPTLC) method was developed for the quantitative assay of these life-saving mixtures in tablets and human plasma. Two mobile phases were developed for the assay in bulk and tablets; the first one: toluene‒ethyl acetate‒methanol‒25% ammonia (3.5:4.5:2:0.2, V/V) (method I) used for the three mixtures, and the second one: methanol‒25% ammonia (9.95:0.05, V/V) (method II) used for EDX/ROS mixture only. For analysis in human plasma, APX was used as internal standard in RIV/ROS and EDX/ROS mixtures using methods I and II, respectively, while RIV was used as internal standard in APX/ROS mixture using method I; the methods were validated according to the Food and Drug Administration (FDA) regulation for analysis in biological fluids. The method selectivity was demonstrated by its ability to simultaneously analyze the drugs in the presence of dosage form excipients and in the presence of plasma interferences (analysis in biological fluid) at single wavelength (291 nm) by use of the internal standard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
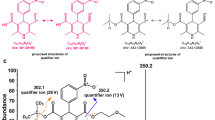
One of the important protocols for prophylaxis in cardiovascular patients to prevent stroke is the combination of novel oral anticoagulants (NOACs) (direct factor Xa inhibitors) with lipid-lowering drugs as statins [1]. According to the World Health Organization (WHO) [2], cardiovascular patients are of very high risk to recurrent stroke that may lead to death and this needs early detection and management [1]. NOACs are indicated for the prevention of recurrent stroke in patients with non-valvular atrial fibrillation [1]. Treatment with a statin is recommended for the primary prevention of ischemic stroke in patients estimated to have a high risk for cardiovascular events [1]. NOACs as apixaban (APX) (Fig. 1a), edoxaban tosylate (EDX) (Fig. 1b) and rivaroxaban (RIV) (Fig. 1c) are direct factor Xa inhibitors in the coagulation cascade, they are used in approved treatment of venous thromboembolism and in the prophylaxis from stroke for patients suffering from atrial fibrillation and pulmonary embolism [, , 1, 3, 4]. Rosuvastatin calcium (ROS) (Fig. 1d) is an antihyperlipidemic drug that belongs to the statin class of medications that competitively inhibits the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase [4]. Statins are used to lower the risk of cardiovascular disease and manage elevated lipid levels, by inhibiting the endogenous production of cholesterol in the liver, thus treatment of hypercholesterolemia and stroke prophylaxis [, 1, 5]. Pharmacovigilance studies and real studies on NOACs are revealing a high incidence of serious bleeding events that often require hospitalization and this demands regular plasma monitoring of NOACs [6].
Some high-performance thin-layer chromatography (HPTLC) methods are reported for the assay of NOACs and ROS either alone or in combination with other drugs in bulk & pharmaceutical formulations [, , , , , , , 7, 8, 9, 10, 11, 12, 13, 14]. RIV is assayed using HPTLC in human plasma [15] and also HPTLC is used to quantify ROS in rabbit plasma [16]. Most bioanalytical techniques available in the literature to quantify NOACs are based on liquid chromatography in human plasma; using ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) [, 17, 18], high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) [, , 19, 20, 21], high-performance liquid chromatography–mass spectrometry (HPLC–MS) [22] and high-performance liquid chromatography–diode array detector (HPLC–DAD) [23].
Most recent assays of ROS either alone or in combination with other drugs include reversed-phase high-performance liquid chromatography (RP-HPLC) in pharmaceutical preparations [, , , 24, 25, 26, 27] and biological fluids [27] and HPLC–MS/MS in both pharmaceutical preparations and biological fluids [27].
The main objective of the present work is to develop the first high-performance thin-layer chromatography (HPTLC) method for the simultaneous assay of ROS with APX (mixture 1), with EDX (mixture 2) and with RIV (mixture 3) in pharmaceutical preparations and human plasma. The validation of the assay in plasma is in accordance with the available guidelines of the Food and Drug Administration (FDA) [28] and the assay in bulk and pharmaceutical preparations is validated according to the guidelines of the International Council for Harmonization (ICH) [29].
2 Experimental
2.1 Instrumentation
TLC plates (20 × 20 cm, aluminum plates with 250-μm thickness, pre-coated with silica gel 60 F254) were purchased from E. Merck (Darmstadt, Germany). The samples were applied to the plates using a 100-μL CAMAG (Muttenz, Switzerland) microsyringe (Hamilton, Bonaduz, Switzerland) in the form of bands using a Linomat IV applicator (CAMAG). The slit dimension was kept at 5.00 × 0.45 mm, and 20 mm s–1 scanning speed was employed. Ascending development of the mobile phase was carried out in a CAMAG 20 cm × 20 cm twin-trough glass chamber. The optimized chamber saturation time for mobile phase was 10 min at room temperature (25 ± 2 °C). Densitometric scanning was performed at 291 nm on a CAMAG TLC Scanner 3 operated in the reflectance–absorbance mode and controlled by CAMAG CATS software (V 3.15). The source of radiation utilized was a mercury lamp emitting a continuous ultraviolet (UV) spectrum between 200 and 400 nm.
2.2 Materials and reagents
Analytical grades of APX, EDX and RIV were kindly supplied by the European Egyptian Pharmaceutical Industries (Alexandria, Egypt) and ROS was kindly supplied by Pharaonia Pharmaceuticals (Cairo, Egypt) with purity % not less than 99.00%. Ammonia (25%) (Adwik El Nasr Chemicals, Giza, Egypt), toluene (Fisher Chemical, Waltham, MA, USA; HPLC grade), ethyl acetate (Adwik El Nasr Chemicals) and methanol (Fisher Chemical, HPLC grade) were used. Fresh blood plasma was supplied from El Madina El Tebya Hospital by the Blood Bank (Sharkya Governerate, Egypt).
2.3 Preparation of standard solutions
2.3.1 Preparation of stock solutions
Standard solutions containing 500 μg mL−1 of each of APX, EDX, RIV and ROS were separately prepared by dissolving the reference materials in methanol and adjusting the volumes. The stock solutions were stored at 4 °C in amber glass vessels and were found to be stable for at least 10 days. All ROS solutions were protected from light.
2.3.2 Preparation of working solutions for assessment of linearity
The working solutions were prepared by dilution of the corresponding stock solutions with methanol. Different volumes from each drug stock solution (ranging from 0.1 to 0.9 mL in 0.1 mL increments) were diluted with methanol in 10-mL volumetric flasks to prepare calibration working solutions in the range of 5‒45 μg mL−1 for the four drugs.
2.4 General procedure and HPTLC conditions for construction of calibration curves
The TLC plates were divided into two halves to obtain plates with dimensions 20 cm × 10 cm. Two different methods were developed for the separation. From each working standard solution, 20-μL portions were spotted on the plates as separate compact bands of 5 mm width, 5 mm apart, 10 mm from the bottom of the plates and 5 mm indented from each side, to obtain final concentrations of 5‒45 μg mL−1 for APX and ROS (mixture 1), RIV and ROS (mixture 3) which were analyzed using method I and for EDX and ROS (mixture 2) assayed using both methods I and II. Triplicate applications were made for each solution and the plates were allowed to air dry for 5 min. The sample-loaded plates were then developed using toluene‒ethyl acetate‒methanol‒25% ammonia (3.5:4.5:2:0.2, V/V) as the mobile phase (method I) for the three mixtures and using methanol‒25% ammonia (9.95:0.05, V/V) (method II) for mixture 2. The plates were developed with the mobile phases over a distance of 93 mm in the usual ascending manner. After development, the plates were air-dried for 5 min and scanned at 291 nm. The peak areas were plotted against the corresponding concentrations to generate the calibration graphs for each compound.
2.5 Analysis of laboratory prepared synthetic mixtures
Five mixtures containing different ratios of the cited drugs were prepared by transferring accurate aliquots from their working solutions into three series of 10-mL volumetric flasks and then the volumes were adjusted to the mark with methanol to reach concentration ranges 5‒45 μg mL−1. The procedure was then completed as mentioned under "General procedures and HPTLC conditions for construction of calibration curves".
2.6 Application of the proposed method to pharmaceutical preparations
Accurately weighed amounts from powdered Eliquis® tablets equivalent to 25 mg APX and from powdered Crestor® tablets equivalent to 50 mg ROS were transferred to a 100-mL volumetric flask using methanol. The flask was sonicated for 30 min, and the volume was adjusted to the mark with the same solvent. The solution was filtered through Whatman No. 1 filter paper.
Similarly, accurately weighed amounts from powdered Lixiana® tablets equivalent to 45 mg EDX and from powdered Crestor® tablets equivalent to 30 mg ROS were transferred to a 100-mL volumetric flask using methanol. The flask was sonicated for 30 min, and the volume was adjusted to the mark with the same solvent. The solution was filtered through Whatman No. 1 filter paper.
Also, accurately weighed amounts from powdered Xarelto® tablets equivalent to 50 mg RIV and from powdered Crestor® tablets equivalent to 50 mg ROS were transferred to a 100-mL volumetric flask using methanol. The flask was sonicated for 30 min and the volume was adjusted to the mark with the same solvent. The solution was filtered through Whatman No. 1 filter paper.
Different portions of the filtrate prepared from the three different mixtures were assayed as specified under "General Procedures and HPTLC conditions for construction of calibration curves". Recovered concentrations were calculated from the corresponding calibration graphs.
For standard addition assay, sample solutions from each mixture extract were spiked with aliquots of standard of both compounds to obtain total concentrations within the linearity ranges, then treated as mentioned under "General Procedures and HPTLC conditions for construction of calibration curves". Recovered concentrations were calculated by comparing the analyte response with the increment response attained after the addition of standard.
2.7 Application to spiked human plasma
2.7.1 Preparation of working solutions for linearity in plasma
Accurately measured volumes of 1.2 to 2.8 mL (in 0.2 mL increments) from the drugs stock solutions were transferred to 10-mL volumetric flasks and the volumes were adjusted with methanol.
2.7.2 Preparation of calibration and quality control standards
In a series of disposable polypropylene microcentrifuge tubes (1.5 mL), 25 µL from each working solution of APX and ROS (mixture1) with the internal standard (IS) RIV (25 µL from the working solution prepared using 2 mL), of EDX and ROS working solutions (mixture 2) with the IS APX (25 µL from the working solution prepared using 2 mL) and of RIV and ROS working solutions (mixture 3) with the IS APX (25 µL from the working solution prepared using 2 mL) were spiked to 750 µL plasma, 0.5 mL acetonitrile was added and then the solutions were mixed in a vortex mixer for 10 min. The tubes were centrifuged at 15 000 rpm for 30 min in a cooling centrifuge at ‒4 °C and 20 μL from the clear supernatant layers were spotted on TLC plates for separation. Triplicate applications were made for each solution and calibration curves were constructed.
Quality controls are used to assess the precision and accuracy of an assay and the stability of the samples. Sponsors should prepare QCs in the same matrix as the study samples to be assayed with the validated method. Freshly prepared QCs are recommended for precision and accuracy analyses during method development, as stability data are generally not available at this time. During method validation, QCs evaluate the performance of a method and the stability of an analyte. Performance QCs are included in validation runs to determine the precision and accuracy of the method. Stability QCs evaluate the stability of an analyte under various stress conditions. The LLOQ (lower limit of quantification) defines the method sensitivity and should be determined during method development. The method should be developed and validated such that it will be able to meet the requirements necessary for the intended study samples. The LLOQ evaluation can be done separately or as part of the precision and accuracy assessment for the calibration range [28].
The calibration and quality control (QC) standards were prepared by spiking plasma with standard solutions. Calibration standards for mixture 1 using method I were prepared at 1.132, 1.32, 2.075, 2.264, 2.45 and 2.64 μg mL−1 for both ROS and APX. QC standards were prepared at 1.1320 (LLOQ), 1.320 (low quality control, LQC), 1.887 (medium quality control, MQC) and 2.64 μg mL−1 (high quality control, HQC) for both APX and ROS. Calibration standards for mixture 3 using method I were prepared at 1.132, 1.32, 1.509, 1.698, 1.8867, 2.075, 2.264, 2.45 and 2.64 μg mL−1 for both ROS and RIV. QC standards were prepared at 1.1320 (LLOQ), 1.320 (LQC), 1.8867 (MQC) and 2.64 μg mL−1 (HQC) for both RIV and ROS. Finally, for mixture 2, using method II, calibration standards were prepared at 1.132, 1.32, 2.075, 2.264, 2.4, 2.64 and 2.83 μg mL−1 for both ROS and EDX. QC standards were prepared at 1.1320 (LLOQ), 1.320 (LQC), 2.264 (MQC) and 2.83 μg mL−1 (HQC) for both ROS and EDX.
3 Results and discussion
3.1 Optimization of HPTLC conditions
The experimental conditions for the HPTLC method such as mobile phase composition and wavelength of detection were optimized to provide accurate, precise, reproducible and compact flat bands for the three drug mixtures.
3.1.1 Solvent system
The mobile phase is regarded as the most important factor that controls the peak shape and resolution; therefore, different solvent systems with different proportions were investigated before reaching the optimum mobile phase. Greener systems were first tried such as methanol with a little proportion of ammonia (9.95:0.05, V/V), but poor resolution was observed for mixtures 1 and 3, while well-separated and well-defined peaks were obtained for mixture 2. A system consisting of ethanol‒chloroform in different proportions was tried, but overlapped and distorted peaks were obtained. Increasing the ratio of chloroform led to poor resolution, while increasing the ratio of ethanol resulted in more distortion of the peaks, so, there was no improvement by changing the ratio of these two solvents. Finally, sharp and symmetric peaks were obtained for the three mixtures using toluene‒ethyl acetate‒methanol‒25% ammonia (3.5:4.5:2:0.2, V/V). The distance traveled by the developed APX, EDX, RIV and ROS bands increased by adding very small volumes of ammonia, thus, the differences between the retardation factor (RF) values of the APX and ROS bands and the RIV and ROS bands increased to reach values of about 0.50 and 0.60, respectively; in addition, the incorporation of ammonia helped in decreasing the tailing effect.
Well-defined bands for the two drugs in each mixture were obtained when the chamber was saturated with the mobile phase at room temperature for at least 30 min. It was required to eliminate the edge effect and to avoid unequal solvent evaporation losses from the developing plate that could lead to behavior resulting in a lack of RF values reproducibility.
3.1.2 Scanning and detection wavelength
Different scanning wavelengths were investigated and 291 nm was chosen for the three mixtures, as it gave reasonable response with all of them. The optimum bandwidth chosen was 5 nm, taking into consideration the range of concentrations applied and number of tracks. All tracks were scanned efficiently at the same wavelength (291 nm) for the three mixtures.
The optimized chromatographic conditions gave compact spots for the cited drugs at the specific RF values which were found to be 0.65 ± 0.01 for APX, 0.20 ± 0.01 for EDX, 0.75 ± 0.02 for RIV and 0.10 ± 0.01 for ROS using method I and 0.40 ± 0.02 for EDX and 0.90 ± 0.02 for ROS using method II. Typical densitograms obtained from the analysis of the mentioned mixtures at the selected wavelength using the proposed methods are shown in Figs. 2, 3, 4, and 5.
3.2 Method validation
For the analysis of the mixtures in bulk and in dosage forms the methods were validated according to the ICH guidelines [29], while for the quantitation of the drugs spiked in human plasma, validation was done according to the FDA guidelines (2001): Guidance for Industry on Bioanalytical Method Validation [28].
3.2.1 Analysis in bulk form and tablets
3.2.1.1 Linearity
Linearity was evaluated by analyzing a series of different concentrations of each of APX and ROS (mixture 1) and RIV and ROS (mixture 3) by applying method I and mixture 2 (EDX and ROS) by applying both methods I and II. Under the experimental conditions previously described, the graphs obtained by plotting peak areas of the drugs versus concentrations in the ranges stated in Table 1 showed linear relationships. The slopes, intercepts and correlation coefficients obtained by the linear least-squares regression treatment of the results are also given. The smaller the standard error of the estimate (Sy/x) obtained, the closer the points are to the straight line. The high values of correlation coefficients and F indicate the good linearity of the calibration curves.
3.2.1.2 Limit of detection and limit of quantitation
The limit of detection (LOD) is considered as the concentration which has a signal-to noise ratio of 3:1. For the limit of quantitation (LOQ), the ratio considered is 10:1 [30]. Using the proposed methods, LOD and LOQ for each compound were calculated and are presented in Table 1. These values were calculated using the signal-to-noise ratio method. Both LOD and LOQ values indicate that the proposed method showed low noise levels along with the high drugs responses which enable the quantitation and detection of low concentrations. The LOD and LOQ values ranged from 0.0022–0.0282 to 0.0074–0.094 μg band–1, respectively.
3.2.1.3 Accuracy and precision
Accuracies either with intra-day or inter-day precision were evaluated using three concentration levels (n = 3) within the same day or on three consecutive days, respectively. The percentage relative standard deviation (RSD%) and percentage relative error (Er%) did not exceed 2.0% proving the high repeatability and accuracy of the developed method for the estimation of the analytes in their bulk form (Tables S1‒S4).
3.2.1.4 Selectivity
Method selectivity was checked by analyzing the laboratory-prepared synthetic mixtures containing different ratios of drugs in each mixture, where good percentage recoveries were obtained indicating that they did not interfere with each other (Tables S5‒S7). In addition, the selectivity of each of the proposed methods was confirmed by the absence of interference from adjuvants during the application to the analysis of pharmaceutical preparations. Figures S8‒S11 present TLC densitograms of the prepared tablet extracts for the three mixtures recorded at 291 nm. These densitograms showed no interfering peaks from the added excipients thus confirming the specificity of the proposed HPTLC methods.
3.2.1.5 Robustness
The robustness of the proposed method was assessed by slightly varying some parameters such as the time of saturation (30 min ± 2 min) and the detection wavelength (± 2 nm). It was found that small deliberate variations in the above parameters had no significant influence on the determination of any of the drugs using the proposed method. The low values of RSD% of the peak areas along with nearly unchanged RF values obtained after introducing small deliberate changes in the method parameters indicated the robustness of the developed method.
3.2.1.6 Stability of solutions
The stability of the standard solutions was investigated over 4 h, where the RF values and the peak areas were unchanged throughout the analysis time. In addition, no extra peaks were observed in the densitograms, confirming the stability of the working solutions. Also, the methanolic stock solutions of the four drugs were found to be stable for at least one week when refrigerated at 4 °C.
3.2.2 Analysis in spiked human plasma
3.2.2.1 Linearity and LLOQ
Method I
For mixtures 1 and 3, the calibration curves of the drugs were constructed from a blank sample (plasma sample processed without IS) (Figs. 6a and 7a), a zero calibrator sample (plasma sample processed with IS) (Figs. 6b and 7b) and non-zero calibration standards encompassing the entire range including LLOQ (Figs. 6c and 7c). Linearity was assessed by the IS method. The calibration curves were linear and the data of regression analysis showed good linearity as the correlation coefficient values were higher than 0.998. Regression equations were Y = − 0.13 + 0.60X and Y = 0.09 + 0.05X, for APX and ROS (mixture 1) and Y = − 0.08 + 0.27X and Y = 0.06 + 0.13X, for RIV and ROS (mixture 3), respectively (Y is the peak area ratio of drug; X is the drug concentration in μg mL–1).
Method 2
For mixture 2, the calibration curves of the drugs were constructed from a blank sample (plasma sample processed without IS) (Fig. 8a), a zero calibrator sample (plasma sample processed with IS) (Fig. 8b) and non-zero calibration standards encompassing the entire range including LLOQ (Fig. 8c). Linearity was assessed by the IS method. The calibration curves were linear and the data of regression analysis showed good linearity as the correlation coefficient values were higher than 0.998. Regression equations were: Y = 1.30 + 1.05X and Y = 0.30 + 0.13X, for EDX and ROS (mixture 2), respectively (Y is the peak area ratio of drug; X is the drug concentration in μg mL–1).
3.2.2.2 Accuracy and precision
The validation batch used consisted of one set of calibration standards and six replicates (n = 6) of quality control samples at four levels (LLOQ, LQC, MQC and HQC). Accuracy and precision were evaluated as described under analysis in bulk form and tablets (RSD%) ranging between 1.00–1.89% and 1.21–2.44% for APX and ROS, respectively (mixture 1); between 0.48–2.90% and 1.24–2.44% for EDX and ROS, respectively (mixture 2); and between 1.16–2.41% and 1.53–2.75% for RIV and ROS, respectively (mixture 3) (Table 2).
3.2.2.3 Specificity
The specificity of the proposed methods was evaluated by processing control plasma from six different packets. The plasma samples were spiked with lower limit of quantification (LLOQ) working solutions along with IS to confirm the lack of interference at their RF values. No interfering peaks from endogenous plasma compounds were observed in blank plasma at the RF of analytes and IS. Typical chromatograms for the blank plasma, and plasma spiked with the drugs forming each mixture along with the corresponding internal standard are shown in Figs. 6, 7, and 8.
3.2.2.4 Stability studies
Stability experiments were performed to evaluate the analyte stability in plasma samples (LQC and HQC, n = 6) under different sample analysis conditions. Long-term stability was evaluated after storage of the samples at − 70 °C for 45 days. Short-term stability was assessed after storage of spiked QC samples at ambient temperature for 6 h. Post-preparative stability was assessed after storage at 5 °C for 24 h. Freeze–thaw stability was assessed by analyzing spiked QC samples after five freeze–thaw cycles. For all the stability experiments of analytes and IS in control plasma, excellent % recovery (97.14–103.90%) and RSD% (less than 2.52%) values for mixture 1; % recovery (97.31–103.28%) and RSD% (less than 2.82%) values for mixture 2; and % recovery (97.44–103.89%) and RSD% (less than 2.51%) values for mixture 3 were obtained, thus indicating their stability under different conditions (Table 3).
3.3 Application to the analysis of pharmaceutical preparations
Due to the unavailability of commercial tablets containing these binary mixtures, single component tablets of ROS with each of the NOACs were mixed in ratios simulating their appropriate doses, and extracted with methanol, then spotted on the TLC plates. The lack of foreign peaks in the chromatograms confirmed that inactive ingredients did not interfere in the analysis. Percentage recoveries were calculated for three independently prepared solutions each repeated three times and were found acceptable (Table S12). Owing to the absence of any published method for the determination of these binary mixtures, the standard addition technique was applied by spiking the tablets’ extracts with portions of the standard solution of each drug to obtain total concentrations within the linearity ranges. The obtained % recoveries, RSD% and Er (%) were satisfactory, thus proving the absence of interference from the tablets excipients.
3.4 Application to the analysis of the binary mixtures in human plasma
The suggested HPTLC methods were successfully applied to the analysis of the three binary mixtures in spiked human plasma; method I was used to analyze mixtures 1 and 3, while method II was used for mixture 2. The procedure adopted was based on simple protein precipitation with acetonitrile with no need for tedious extraction steps (Figs. 6, 7, 8).
3.5 Assessment of method greenness
Green and eco-friendly practices have been recently adopted in different analytical procedures such as using green sample pretreatment, using environmentally friendly solvents and reagents, consuming less energy and shortening analysis times.
Greenness assessment was done using two different recent methods. The Green Analytical Procedure Index [31] (GAPI), a widely cited green assessing method newly introduced in 2018 was used. The GAPI pectogram is represented by five major pentagrams divided into 15 zones where each pentagram represents a step in the analytical procedure. These zones are colored by three colors (green, yellow and red) used in the greenness assessment, where theses colors represents low, moderate and high impact on the environment, respectively. As shown in Table 4, GAPI pictogram shows only one red zone which represents the off-line sampling since the sites of production of the pharmaceutical preparations must be away from the laboratories where the HPTLC method was designed. Such a pictogram indicates that the method is a good green method for the analysis of these binary mixtures. Also, Analytical GREEnness Metric Approach [32] (AGREE), a new method introduced in 2020, was used. AGREE assessment is based on the twelve principles of Green Analytical Chemistry (GAC) and is represented in a clock-like graph composed of twelve sections representing the twelve GAC principles. Each section is assessed and represented in a color of green, yellow and red. The overall greenness performance of the twelve sections is written in the middle of the clock-like graph with a score within 0–1 and with a color. As shown in Table 4, the clock-like graph shows an overall AGREE score of 0.74 with green color indicating the low impact on the environment. Only one red was found representing the off-line sampling that occurs in the analytical quality control laboratories of pharmaceuticals.
4 Conclusion
The proposed HPTLC methods developed for the determination of binary mixtures of the co-administered lipid-lowering agent ROS and the NOACs APX, EDX and RIV are simple and sensitive. To the best of our knowledge, this is the first validated HPTLC method developed to simultaneously quantify these three binary mixtures in pharmaceutical preparations and human plasma. Simplicity was demonstrated by the absence of lengthy sample pretreatment procedures. Sensitivity was confirmed by the ability of the methods to analyze these mixtures in spiked plasma samples in concentrations simulating the real ones, taking into consideration the extensive binding to plasma proteins that these drugs exhibit. The greenness of the method was proved by two powerful metric scales, the GAPI and AGREE metric methods. In addition, TLC, as analytical technique, provides rapid and cost-effective analysis, thus allowing a high sample throughput in a single run and minimum consumption of relatively cheap solvents.
Data availability
All data generated or analyzed during this study are included in this published article. Any further data can be requested from the corresponding author.
References
Sherzai AZ, Elkind MSV (2015) Advances in stroke prevention. Ann N Y Acad Sci 1338:1–15. https://doi.org/10.1111/nyas.12723
World Health Organization (WHO) (2021) Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 14 January 2022
Verheugt FWA, Granger CB (2015) Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations and unmet needs. Lancet 386:303–310. https://doi.org/10.1016/S0140-6736(15)60245-8
Sweetman SC (2014) Martindale: The Complete Drug Reference, 38th edn. The Pharmaceutical Press, London
Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med 5:378–387. https://doi.org/10.1111/j.1582-4934.2001.tb00172.x
Garbayo JLM, Canada MK, Castelló IP, Faus MTS, Ribis MP (2019) Hospital admissions for bleeding events associated with treatment with apixaban, dabigatran and rivaroxaban. Eur J Hosp Pharm 26:106–112. https://doi.org/10.1136/ejhpharm-2017-001390
Dhiware TK, Patil PA, Salaraya MG (2019) Development and Validation of HPTLC Method for Determination of Edoxaban in Bulk and Tablet. Asian J Pharm Anal 9:161–166. https://doi.org/10.5958/2231-5675.2019.00029.2
Jain HK, Nika VK (2017) Development and validation of HPTLC method for determination of apixaban in bulk and tablets. Int J App Pharm 9:78–83. https://doi.org/10.22159/ijap.2017v9i5.20028
Daml MC, Waghmare SS, Sinha P (2019) Development and Validation of Stability Indicating HPTLC Method for determination of Apixaban as Bulk Drug. Int J Pharm Pharm Sci 11:37–42. https://imsear.searo.who.int/handle/123456789/205895
Alam P, Ezzeldin E, Iqbal M, Anwer MK, Mostafa GA, Alqarni MH, Foudah AI, Shakeel F (2020) Ecofriendly densitometric RP-HPTLC method for determination of rivaroxaban in nanoparticle formulations using green solvents. RSC Adv 10:2133–2140. https://doi.org/10.1039/C9RA07825H
Khurd AS, Doshi KV (2019) Quality by Design-Based Optimization and Validation of a High-Performance Thin-Layer Chromatography Method for the Estimation of Rivaroxaban in Bulk and Its Pharmaceutical Dosage Form. JPC – J Planar Chromat 32:505–510. https://doi.org/10.1556/1006.2019.32.6.9
Patil PM, Wawdhane S, Chaudhari P (2017) Identification, separation and characterization of acidic and alkali degradation of rivaroxaban under ICH recommended stress condition by HPTLC with MS/TOF. Anal Chem Lett 7:706–723. https://doi.org/10.1080/22297928.2017.1375980
Sane R, Kamat RS, Menon S, Inamdar S, Mote M (2005) Determination of rosuvastatin calcium in its bulk drug and pharmaceutical preparations by high-performance thin-layer chromatography. JPC – J Planar Chromat 18:194–198. https://doi.org/10.1556/jpc.18.2005.3.4
Sankar AS, Shanmugasundaram P, Ravichandiran V (2017) A robust QBD based high-performance thin-layer chromatographic method for the estimation of aspirin, clopidogrelbisulphate and rosuvastatin calcium in a pharmaceutical formulation using experimental design. Curr Anal Chem 13:386–392. https://doi.org/10.2174/1573411013666170127163707
Shukla AH, Shah PJ, Dedhiya PP, Vyas BA, Shah SA (2020) Development and validation of a HPTLC method for rivaroxaban in human plasma for a pharmacokinetic study. Ind J Pharm Sci 82:315–320. https://doi.org/10.36468/pharmaceutical-sciences.652
Abbas NS, Derayea SM, Omar MA, Saleh GA (2020) TLC-spectrodensitometric method for simultaneous determination of dapagliflozin and rosuvastatin in rabbit plasma: stability indicating assay and kinetic studies. RSC Adv 10:40795–40805. https://doi.org/10.1039/D0RA05628F
Lindahl S, Dyrkorn R, Spigset O, Hegstad MDS (2018) Quantification of Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in Human Serum by UHPLC-MS/MS—Method Development, Validation, and Application. Ther Drug Monit 40:369–376. https://doi.org/10.1097/FTD.0000000000000509
Foerster KI, Huppertz A, Müller OJ, Rizos T, Tilemann L, Haefeli WE, Burhenne J (2018) Simultaneous quantification of direct oral anticoagulants currently used in anticoagulation therapy. J Pharm Biomed Anal 148:238–244. https://doi.org/10.1016/j.jpba.2017.10.011
Blaich C, Müller C, Michels G, Wiesen MHJ (2015) Multi-analyte analysis of non-vitamin K antagonist oral anticoagulants in human plasma using tandem mass spectrometry. Clin Chem Lab Med 53:1981–1990. https://doi.org/10.1515/cclm-2014-1108
Zhang M, Moore GA, Chin PKL (2020) Simultaneous Determination of Dabigatran, Rivaroxaban, and Apixaban in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry. Ther Drug Monit 42:473–480. https://doi.org/10.1097/FTD.0000000000000744
Lagoutte-Renosi J, Le Poupon J, Girard A, Montange D, Davani S (2018) A simple and fast HPLC-MS/MS method for simultaneous determination of direct oral anticoagulants apixaban, dabigatran, rivaroxaban in human plasma. J Chromatogr B 1100–1101:43–49. https://doi.org/10.1016/j.jchromb.2018.09.026
Gous T, Couchman L, Patel JP, Paradzai C, Arya R, Flanagan RJ (2014) Measurement of the direct oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in human plasma using turbulent flow liquid chromatography with high-resolution mass spectrometry. Ther Drug Monit 36:597–605. https://doi.org/10.1097/FTD.0000000000000059
Gouveia F, Bicker J, Santos J, Rocha M, Alves G, Falcão A, Fortuna A (2020) Development, validation and application of a new HPLC-DAD method for simultaneous quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. J Pharm Biomed Anal 181:113109. https://doi.org/10.1016/j.jpba.2020.113109
Janardhanan VS, Manavalan R, Valliappan K (2017) Chemometric technique for the optimization of chromatographic system: Simultaneous HPLC determination of Rosuvastatin, Telmisartan, Ezetimibe and Atorvastatin used in combined cardiovascular therapy. Arab J Chem 9:S1378–S1387. https://doi.org/10.1016/j.arabjc.2012.03.001
El-Abasawi NM, Attia KA, Abo-serie AA, Abdel-Fattah A (2018) Simultaneous determination of rosuvastatin and propranolol by HPLC in pharmaceutical dosage form. Anal Chem Lett 8:46–53. https://doi.org/10.1080/22297928.2017.1396919
Pimpale A, Kakde R (2020) Development and Validation of Stability-Indicating Assay Method by RP-HPLC for Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Pharmaceutical Dosage Form. J Drug Deliv Ther 10:79–86. https://doi.org/10.22270/jddt.v10i4.4203
Ângelo ML, Moreira FD, MoraisRuela AL, Santos AL, Salgado HR, de Araújo MB (2018) Analytical methods for the determination of rosuvastatin in pharmaceutical formulations and biological fluids: A critical review. Crit Rev Anal Chem 48:317–329. https://doi.org/10.1080/10408347.2018.1439364
Food and Drug Administration (FDA) (2018) Guidance for Industry: Bioanalytical Method Validation. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed: 14 January 2022)
International Conference on Harmonization (ICH) (2005) Harmonized Tripartite Guideline Validation of Analytical Procedures, Text and Methodology, Q2 (R1). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed: 14 January 2022)
The United States Pharmacopeia (2018) 41st edition, The National Formulary, 36th edn. United States Pharmacopeial Convention Inc, Asian Edition, Washington, DC
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness Metric Approach and Software. Anal Chem 92:10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that this research is not funded from any source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdy, M.M.A., Korany, M.A., Ebied, S.A. et al. ICH and US-FDA validated HPTLC methods with greenness assessments for the assay of mixtures prescribed in stroke prophylaxis: application to pharmaceutical preparations and human plasma. JPC-J Planar Chromat 35, 519–532 (2022). https://doi.org/10.1007/s00764-022-00201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-022-00201-4