Abstract
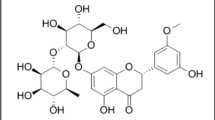
The lipase inhibitory activities of four main components from the rhizomes of Alisma orientale (Sam.) Juz. were evaluated by an in situ high-performance thin-layer chromatography (HPTLC)‒bioautographic assay taking orlistat as control standard. The order of relative activity was alisol B 23-acetate > alisol B > alisol A > alisol C 23-acetate. With that, an accurate, efficient and sustainable HPTLC method was developed to simultaneously determine the four lipase inhibitors from the methanolic extracts of Alismatis Rhizoma (AR). The method was carried out on HPTLC glassed plates (20 × 10 cm) coated with silica gel 60 F254 (0.2 mm thickness) using a mixture of cyclohexane and ethyl acetate (1:1, V/V) as the mobile phase. The RF values found for alisol B 23-acetate, alisol C 23-acetate, alisol B and alisol A were 0.62, 0.42, 0.28 and 0.09, respectively. The method was validated for specificity, linear range, precision, stability, and recovery. The results determined by scanning densitometry showed no significant difference to the results obtained by HPLC. The developed method was verified to be trustworthy for the evaluation of quality markers in AR.






Similar content being viewed by others
References
Pharmacopoeia Commission of People’s Republic of China (2020) Pharmacopoeia of the People’s Republic of China (Part 1). Chinese Medical Science and Technology Press, Beijing, p 212
Dan H, Wu J, Peng M, Hu XF, Song CW, Zhou ZW, S, Yu SG, N, Fang NB, (2011) Hypolipidemic effects of Alismatis rhizome on lipid profile in mice fed high-fat diet. Saudi Med J 32:701–707 (PMID: 21748207)
Liao ML, Shang HH, Li YZ, Li T, Wang M, Zheng YN, Hou WB, Liu CX (2018) An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network Pharmacology. Phytomedicine 45:93–104. https://doi.org/10.1016/j.phymed.2018.04.006
Feng YL, Chen H, Tian T, Chen DQ, Zhao YY, Lin RC (2014) Diuretic and antidiuretic activities of the ethanol and aqueous extracts of Alismatis rhizome. J Ethnopharmacol 154:386–390. https://doi.org/10.1016/j.jep.2014.04.017
Miao H, Zhang L, Chen DQ, Chen H, Zhao YY, Ma SC (2017) Urinary biomarker and treatment mechanism of Rhizoma Alismatis on hyperlipidemia. Biomed Chromatogr 31:3829. https://doi.org/10.1002/bmc.3829
Li Q, Qu HB (2012) Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma. Fitoterapia 83:1046–1053. https://doi.org/10.1016/j.fitote.2012.05.009
Tian T, Chen H, Zhao YY (2014) Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: a review. J Ethnopharmacol 158:373–387. https://doi.org/10.1016/j.jep.2014.10.061
Ma QJ, Han L, Bi XX, Wang XB, Mu Y, Guan PP, Li LY, Huang XS (2016) Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale. Phytochemistry 131:150–157. https://doi.org/10.1016/j.phytochem.2016.08.015
Ho CK, Gao Y, Zheng DN, Liu YJ, Shan SZ, Fang B, Zhao YX, Song DZ, Zhang YF, Li QF (2019) Alisol A attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J Cell Mol Med 23:5108–5118. https://doi.org/10.1111/jcmm.14380
Kanno Y, Yatsu T, Yamashita N, Zhao S, Li W, Imai M, Kashima M, Inouye Y, Nemoto K, Koike K (2017) Alisol B 23-acetate from the rhizomes of Alisma orientale is a natural agonist of the human pregnane X receptor. Phytomedicine 26:22–27. https://doi.org/10.1016/j.phymed.2017.01.003
Liu SS, Sheng WL, Li Y, Zhang SS, Zhu JJ, Gao HM, Yan LH, Wang ZM, Gao L, Zhang M (2019) Chemical constituents from Alismatis Rhizoma and their anti-inflammatory activities in vitro and in vivo. Bioorg Chem 92:103226. https://doi.org/10.1016/j.bioorg.2019.103226
Han CW, Kwun MJ, Kim KH, Choi JY, Oh SR, Ahn KS, Lee JH, Joo MS (2013) Ethanol extract of Alismatis Rhizoma reduces acute lung inflammation by suppressing NF-κB and activating Nrf2. J Ethnopharmacol 46:402–410. https://doi.org/10.1016/j.jep.2013.01.010
Yang F, Gu LH, Han ZZ, Wang ZT (2021) Rapid screening for natural lipase inhibitors from Alisma orientale combining high-performance thin-layer chromatography-bioautography with mass spectrometry. J Chromatogr B 1170:122599. https://doi.org/10.1016/j.jchromb.2021.122599
Tai YN, Wu X, Fan LM, Wu ZN, Weng YH, Lin QQ (2018) Simultaneous determination of sixteen components in Alismatis Rhizoma by UPLC–MS/MS. Chin J Pharm Anal 38:1337–1350. https://doi.org/10.16155/j.0254-1793.2018.08.08
Zhang YW, Li Q, Lv CX, Liu XJ, Chen XH, Bi KS (2015) Simultaneous determination of four active components in Alisma orientale (Sam.) Juz. by HPLC–DAD using a single reference standard. J Pharm Anal 5:85–92. https://doi.org/10.1016/j.jpha.2014.12.001
Tian SS, Zhao XM, Liu SS, Feng WH, Chen LM, Yan LH, Wang ZM (2020) Regional differences analysis of Alismatis Rhizoma based on UPLC characteristic chromatogram and determination of eight terpenoids. China J Chin Mater Med 45:1545–1557. https://doi.org/10.19540/j.cnki.cjcmm.20200324.201
Zhao WL, Huang XQ, Li XY, Zhang FF, Chen SN, Ye M, Huang MQ, Xu W, Wu SS (2015) Qualitative and quantitative analysis of major, triterpenoids in Alismatis Rhizoma by high performance liquid chromatography/diode-array detector/quadrupole-time-of-flight mass spectrometry and ultraperformance liquid chromatography/triple quadrupole mass spectrometry. Molecules 20:13958–13981. https://doi.org/10.3390/molecules200813958
Klingelhöfer I, Morlock GE (2019) Lovastatin in lactone and hydroxy acid forms and citrinin in red yeast rice powders analyzed by HPTLC–UV/FLD. Anal Bioanal Chem 411:6655–6665. https://doi.org/10.1007/s00216-019-02039-y
Liu MT, Zhao J, Li SP (2021) Application of smartphone in detection of thin-layer chromatography: Case of salvia miltiorrhiza. J Chromatogr A 1637:461826. https://doi.org/10.1016/j.chroma.2020.461826
Abdelhamid NS, Naguib IA, Anwar BH, Magdy MA (2020) A validated HPTLC method for the quantitative determination of duloxetine hydrochloride and 1-naphthol in bulk and pharmaceutical formulation. J Planar Chromat 33:391–396. https://doi.org/10.1007/s00764-020-00045-w
Xu LJ, Liu SB (2021) Forecasting structure of natural products through color formation process by thin layer chromatography. Food Chem 334:127496. https://doi.org/10.1016/j.foodchem.2020.127496
Gu LH, Liao LP, Hu HJ, Bligh SWA, Wang CH, Chou GX, Wang ZT (2015) A thin-layer chromatography-bioautographic method for detecting dipeptidyl peptidase IV inhibitors in plants. J Chromatogr A 1411:116–122. https://doi.org/10.1016/j.chroma.2015.07.123
Acknowledgements
This study was financially supported by a project from Shanghai Natural Science Fund (No. 19ZR1457200) and a project from Chinese Pharmacopoeia Commission (No. 2018Z003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, F., Kim, M., Gu, L. et al. Stimulation quantification of four natural lipase inhibitors from Alismatis Rhizoma by high-performance thin-layer chromatography method. JPC-J Planar Chromat 35, 3–12 (2022). https://doi.org/10.1007/s00764-022-00152-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-022-00152-w