Abstract
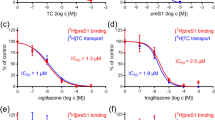
Bile acid prodrugs have served as a viable strategy for refining the pharmaceutical profile of parent drugs through utilizing bile acid transporters. A series of three ester prodrugs of the antiherpetic drug acyclovir (ACV) with the bile acids cholic, chenodeoxycholic and deoxycholic were synthesized and evaluated along with valacyclovir for their in vitro antiviral activity against herpes simplex viruses type 1 and type 2 (HSV-1, HSV-2). The in vitro antiviral activity of the three bile acid prodrugs was also evaluated against Epstein–Barr virus (EBV). Plasma stability assays, utilizing ultra-high performance liquid chromatography coupled with tandem mass spectrometry, in vitro cytotoxicity and inhibitory experiments were conducted in order to establish the biological profile of ACV prodrugs. The antiviral assays demonstrated that ACV-cholate had slightly better antiviral activity than ACV against HSV-1, while it presented an eight-fold higher activity with respect to ACV against HSV-2. ACV-chenodeoxycholate presented a six-fold higher antiviral activity against HSV-2 with respect to ACV. Concerning EBV, the highest antiviral effect was demonstrated by ACV-chenodeoxycholate. Human plasma stability assays revealed that ACV-deoxycholate was more stable than the other two prodrugs. These results suggest that decorating the core structure of ACV with bile acids could deliver prodrugs with amplified antiviral activity.





Similar content being viewed by others
Abbreviations
- ACV:
-
Acyclovir
- HSV-1:
-
Herpes simplex virus type 1
- HSV-2:
-
Herpes simplex virus type 2
- EBV:
-
Epstein–Barr virus
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- RT:
-
Retention time
- ΜRΜ:
-
Multiple reaction monitoring
- MTC:
-
Maximum tolerable concentration
- MIC:
-
Minimum inhibitory concentration
References
Antman MD, Gudmundsson OS (2007) Case study: valacyclovir: a prodrug of acyclovir. In: Stella VJ, Borchardt RT, Hageman MJ, Oliyai R, Maag H, Tilley JW (eds) Prodrugs: challenges and rewards part 1. Springer, New York, pp 1369–1376. https://doi.org/10.1007/978-0-387-49785-3_54
Arkin LM, Castelo-Soccio L, Kovarik C (2009) Disseminated herpes simplex virus (HSV) hepatitis diagnosed by dermatology evaluation. Int J Dermatol 48(9):1020–1021. https://doi.org/10.1111/j.1365-4632.2009.04084.x
Balakrishnan A, Polli JE (2006) Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol Pharm 3(3):223–230. https://doi.org/10.1021/mp060022d
Bando H, Yamashita F, Takakura Y, Hashida M (1994) Skin penetration enhancement of acyclovir by prodrug-enhancer combination. Biol Pharm Bull 17(8):1141–1143
Beauchamp LM, Krenitsky TA (1993) Acyclovir prodrugs: the road to valaciclovir. Drugs Future 18(7):619–628
Beauchamp LM, Orr GF, de Miranda P, Bumette T, Krenitsky TA (1992) Amino acid ester prodrugs of acyclovir. Antiviral Chem Chemother 3(3):157–164. https://doi.org/10.1177/095632029200300305
Berger JR, Houff S (2008) Neurological complications of herpes simplex virus type 2 infection. Arch Neurol 65(5):596–600. https://doi.org/10.1001/archneur.65.5.596
Bockman DE, Ganapathy V, Oblak TG, Leibach FH (1997) Localization of peptide transporter in nuclei and lysosomes of the pancreas. Int J Pancreatol 22(3):221–225. https://doi.org/10.1007/BF02788388
Bomgaars L, Thompson P, Berg S, Serabe B, Aleksic A, Blaney S (2008) Valacyclovir and acyclovir pharmacokinetics in immunocompromised children. Pediatr Blood Cancer 51(4):504–508. https://doi.org/10.1002/pbc.21638
Bras AP, Sitar DS, Aoki FY (2001) Comparative bioavailability of acyclovir from oral valacyclovir and acyclovir in patients treated for recurrent genital herpes simplex virus infection. Can J Clin Pharmacol 8(4):207–211
Bruni G, Maietta M, Maggi L, Mustarelli P, Ferrara C, Berbenni V, Milanese C, Girella A, Marini A (2013) Preparation and physicochemical characterization of acyclovir cocrystals with improved dissolution properties. J Pharm Sci 102(11):4079–4086. https://doi.org/10.1002/jps.23721
Burnette TC, de Miranda P (1994) Metabolic disposition of the acyclovir prodrug valaciclovir in the rat. Drug Metab Dispos 22(1):60–64
Carey MC, Cahalane MJ (1988) In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DS (eds) In the liver: biology and pathobiology. Raven Press, New York, pp 573–616
Chaudhary D, Ahmed S, Liu N, Marsano-Obando L (2017) Acute liver failure from herpes simplex virus in an immunocompetent patient due to direct inoculation of the peritoneum. ACG Case Rep J 4:e23. https://doi.org/10.14309/crj.2017.23
Chiang JY (2009) Bile acids: regulation of synthesis. J Lipid Res 50(10):1955–1966. https://doi.org/10.1194/jlr.R900010-JLR200
Colla L, De Clercq E, Busson R, Vanderhaeghe H (1983) Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine). J Med Chem 26(4):602–604. https://doi.org/10.1021/jm00358a029
Davis AP (1993) Cholaphanes et al.; steroids as structural components in molecular engineering. Chem Soc Rev 22(4):243–253. https://doi.org/10.1039/cs9932200243
de Miranda P, Blum MR (1983) Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother 12 Suppl B:29–37
de Miranda P, Krasny HC, Page DA, Elion GB (1981) The disposition of acyclovir in different species. J Pharmacol Exp Ther 219(2):309–315
de Miranda P, Krasny HC, Page DA, Elion GB (1982) Species differences in the disposition of acyclovir. Am J Med 73(1A):31–35
Duane WC, Xiong W, Wolvers J (2007) Effects of bile acids on expression of the human apical sodium dependent bile acid transporter gene. Biochim Biophys Acta 1771(11):1380–1388. https://doi.org/10.1016/j.bbalip.2007.09.003
Elnaggar YS (2015) Multifaceted applications of bile salts in pharmacy: an emphasis on nanomedicine. Int J Nanomed 10:3955–3971. https://doi.org/10.2147/IJN.S82558
Enhsen A, Kramer W, Wess G (1998) Bile acids in drug discovery. Drug Discov Today 3(9):409–418
Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ (1999) Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J Pharmacol Exp Ther 289(1):448–454
Gupta S, Agarwal A, Gupta NK, Saraogi G, Agrawal H, Agrawal GP (2013) Galactose decorated PLGA nanoparticles for hepatic delivery of acyclovir. Drug Dev Ind Pharm 39(12):1866–1873. https://doi.org/10.3109/03639045.2012.662510
Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL (1998) 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res 15(8):1154–1159
Hofmann A (1988) In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA (eds) The liver: biology and pathobiology. Raven Press, New York, pp 553–572
Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, Schiffer JT, Koelle DM, Corey L, Wald A (2012) Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 379(9816):641–647. https://doi.org/10.1016/S0140-6736(11)61750-9
Kagedahl M, Swaan PW, Redemann CT, Tang M, Craik CS, Szoka FC Jr, Oie S (1997) Use of the intestinal bile acid transporter for the uptake of cholic acid conjugates with HIV-1 protease inhibitory activity. Pharm Res 14(2):176–180
Karaman R, Dajani KK, Qtait A, Khamis M (2012) Prodrugs of acyclovir—a computational approach. Chem Biol Drug Des 79(5):819–834. https://doi.org/10.1111/j.1747-0285.2012.01335.x
Katragadda S, Jain R, Kwatra D, Hariharan S, Mitra AK (2008) Pharmacokinetics of amino acid ester prodrugs of acyclovir after oral administration: interaction with the transporters on Caco-2 cells. Int J Pharm 362(1–2):93–101. https://doi.org/10.1016/j.ijpharm.2008.06.018
Kim DC, Harrison AW, Ruwart MJ, Wilkinson KF, Fisher JF, Hidalgo IJ, Borchardt RT (1993) Evaluation of the bile acid transporter in enhancing intestinal permeability to renin-inhibitory peptides. J Drug Target 1(4):347–359. https://doi.org/10.3109/10611869308996094
Kimberlin DW, Whitley RJ (2007) Antiviral therapy of HSV-1 and -2. In: Arvin A, Campadelli-Fiume G, Mocarski E et al (eds) Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge
Klysik K, Pietraszek A, Karewicz A, Nowakowska M (2018) Acyclovir in the treatment of herpes viruses—a review. Curr Med Chem. https://doi.org/10.2174/0929867325666180309105519
Kramer W, Glombik H (2006) Bile acid reabsorption inhibitors (BARI): novel hypolipidemic drugs. Curr Med Chem 13(9):997–1016
Kramer W, Wess G (1996) Bile acid transport systems as pharmaceutical targets. Eur J Clin Invest 26(9):715–732
Kramer W, Wess G, Schubert G, Bickel M, Girbig F, Gutjahr U, Kowalewski S, Baringhaus KH, Enhsen A, Glombik H et al (1992) Liver-specific drug targeting by coupling to bile acids. J Biol Chem 267(26):18598–18604
Kramer W, Wess G, Enhsen A, Falk E, Hoffmann A, Neckermann G, Schubert G, Urmann M (1997) Modified bile acids as carriers for peptides and drugs. J Control Release 46(1):17–30. https://doi.org/10.1016/S0168-3659(96)01599-4
Lack L (1979) Properties and biological significance of the ileal bile salt transport system. Environ Health Perspect 33:79–89
Li Y, Dias JR (1997) Dimeric and oligomeric steroids. Chem Rev 97(1):283–304
Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH (1995) Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem 270(12):6456–6463
Longerich T, Eisenbach C, Penzel R, Kremer T, Flechtenmacher C, Helmke B, Encke J, Kraus T, Schirmacher P (2005) Recurrent herpes simplex virus hepatitis after liver retransplantation despite acyclovir therapy. Liver Transpl 11(10):1289–1294. https://doi.org/10.1002/lt.20567
Love MW, Dawson PA (1998) New insights into bile acid transport. Curr Opin Lipidol 9(3):225–229
Miller G, Lipman M (1973) Release of infectious Epstein–Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA 70(1):190–194
Mukhopadhyay S, Maitra U (2004) Chemistry and biology of bile acids. Curr Sci 87(12):1666–1683
Norvell JP, Blei AT, Jovanovic BD, Levitsky J (2007) Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 13(10):1428–1434. https://doi.org/10.1002/lt.21250
Park G-B, Shao Z, Mitra AK (1992) Acyclovir permeation enhancement across intestinal and nasal mucosae by bile salt-acylcarnitine mixed micelles. Pharm Res 9(10):1262–1267. https://doi.org/10.1023/a:1015845031488
Petzinger E (1994) Transport of organic anions in the liver. An update on bile acid, fatty acid, monocarboxylate, anionic amino acid, cholephilic organic anion, and anionic drug transport. Rev Physiol Biochem Pharmacol 123:47–211
Pyles RB (2001) The association of herpes simplex virus and Alzheimer’s disease: a potential synthesis of genetic and environmental factors. Herpes J IHMF 8(3):64–68
Rais R, Fletcher S, Polli JE (2011) Synthesis and in vitro evaluation of gabapentin prodrugs that target the human apical sodium-dependent bile acid transporter (hASBT). J Pharm Sci 100(3):1184–1195. https://doi.org/10.1002/jps.22332
Santos CR, Capela R, Pereira CSGP, Valente E, Gouveia L, Pannecouque C, De Clercq E, Moreira R, Gomes P (2009) Structure–activity relationships for dipeptide prodrugs of acyclovir: implications for prodrug design. Eur J Med Chem 44(6):2339–2346. https://doi.org/10.1016/j.ejmech.2008.08.009
Shao Z, Hoffman AJ, Mitra AK (1994) Biodegradation characteristics of acyclovir 2′-esters by respiratory carboxylesterases: implications in prodrug design for intranasal and pulmonary drug delivery. Int J Pharm 112(2):181
Sievanen E (2007) Exploitation of bile acid transport systems in prodrug design. Molecules 12(8):1859–1889
Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J (1995) Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother 39(12):2759–2764
Staels B, Fonseca VA (2009) Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32(Suppl 2):S237–S245. https://doi.org/10.2337/dc09-S355
Stedronsky ER (1994) Interaction of bile acids and cholesterol with non-systemic agents having hypocholesterolemic properties. Biochim Biophys Acta 1210(3):255–287
Stojančević M, Pavlović N, Goločorbin-Kon S, Mikov M (2013) Application of bile acids in drug formulation and delivery. Front Life Sci 7(3–4):112–122. https://doi.org/10.1080/21553769.2013.879925
St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ (2001) Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol 204(Pt 10):1673–1686
Swaan PW, Hillgren KM, Szoka FC, Øie S (1997a) Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconj Chem 8(4):520–525. https://doi.org/10.1021/bc970076t
Swaan PW, Szoka FC Jr, Oie S (1997b) Molecular modeling of the intestinal bile acid carrier: a comparative molecular field analysis study. J Comput Aided Mol Des 11(6):581–588
Tamminen J, Kolehmainen E (2001) Bile acids as building blocks of supramolecular hosts. Molecules 6(1):21
Thamotharan M, Lombardo YB, Bawani SZ, Adibi SA (1997) An active mechanism for completion of the final stage of protein degradation in the liver, lysosomal transport of dipeptides. J Biol Chem 272(18):11786–11790
Tolle-Sander S, Lentz KA, Maeda DY, Coop A, Polli JE (2004) Increased acyclovir oral bioavailability via a bile acid conjugate. Mol Pharm 1(1):40–48
Turley DS, Dietschy JM (1988) In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DS (eds) In the liver: biology and pathobiology. Raven Press, New York, pp 617–641
Virtanen E, Kolehmainen E (2004) Use of bile acids in pharmacological and supramolecular applications. Eur J Org Chem 16:3385–3399. https://doi.org/10.1002/ejoc.200300699
Vivian D, Polli JE (2014) Synthesis and in vitro evaluation of bile acid prodrugs of floxuridine to target the liver. Int J Pharm 475(1–2):597–604. https://doi.org/10.1016/j.ijpharm.2014.09.014
Wallimann P, Marti T, Fürer A, Diederich F (1997) Steroids in molecular recognition. Chem Rev 97(5):1567–1608. https://doi.org/10.1021/cr960373b
Wang J, Yan X, Lu R, Meng X, Nie G (2017) Peptide transporter 1 (PepT1) in fish: a review. Aquac Fish 2(5):193–206. https://doi.org/10.1016/j.aaf.2017.06.007
Yang C, Tirucherai GS, Mitra AK (2001) Prodrug based optimal drug delivery via membrane transporter/receptor. Expert Opin Biol Ther 1(2):159–175. https://doi.org/10.1517/14712598.1.2.159
Zhang D, Li D, Shang L, He Z, Sun J (2016) Transporter-targeted cholic acid-cytarabine conjugates for improved oral absorption. Int J Pharm 511(1):161–169. https://doi.org/10.1016/j.ijpharm.2016.06.139
Acknowledgements
We thank Dr. Regina Feederle from the German Cancer Research Center, Heidelberg for assistance with immunofluorescence staining, and Dr. Yavor Mitrev from Laboratory “Bulgarian NMR Centre” Institute of Organic Chemistry with Centre of Phytochemistry, Sofia for helpful discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All authors listed have contributed to conception, design, gathering, analysis, or interpretation of data, and have contributed to the writing and intellectual content of the article. All authors gave informed consent to the submission of this manuscript.
Additional information
Handling Editor: G. J. Peters.
Rights and permissions
About this article
Cite this article
Chayrov, R.L., Stylos, E.K., Chatziathanasiadou, M.V. et al. Tailoring acyclovir prodrugs with enhanced antiviral activity: rational design, synthesis, human plasma stability and in vitro evaluation. Amino Acids 50, 1131–1143 (2018). https://doi.org/10.1007/s00726-018-2590-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2590-y