Abstract
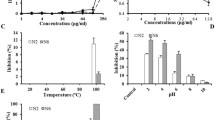
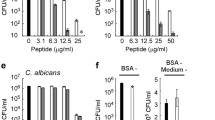
Microcin J25 (MccJ25) is an antibacterial peptide with a peculiar molecular structure consisting of 21 amino acids and a unique lasso topology that makes it highly stable. We synthesized various MccJ25-derived peptides that retained some of the inhibitory activity of the native molecule against Salmonella enterica and Escherichia coli. Of the tested peptides, C1, 7-21C and WK_7-21 were the most inhibitory peptides (MIC = 1–250 µM), but all three were less potent than MccJ25. While MccJ25 was not active against Gram-positive bacteria, the three derived peptides were slightly inhibitory to Gram-positive bacteria (MIC ≥ 250 µM). At 5 µM, C1, 7-21C and WK_7-21 reduced E. coli RNA polymerase activity by respectively, 23.4, 37.4 and 65.0 %. The MccJ25 and its derived peptides all appeared to affect the respiratory apparatus of S. enterica. Based on circular dichroism and FTIR spectroscopy, the peptides also interact with bacterial membrane phospholipids. These results suggest the possibility of producing potent MccJ25-derived peptides lacking the lasso structure.









Similar content being viewed by others
References
Bayro MJ, Mukhopadhyay J, Swapna GVT, Huang JY, Ma L-C, Sineva E, Dawson PE, Montelione GT, Ebright RH (2003) Structure of antibacterial peptide microcin J25: a 21-residue lariat protoknot. J Am Chem Soc 125(41):12382–12383. doi:10.1021/ja036677e
Bellomio A, Rintoul MR, Morero RD (2003) Chemical modification of microcin J25 with diethylpyrocarbonate and carbodiimide: evidence for essential histidyl and carboxyl residues. Biochem Biophys Res Commun 303(2):458–462
Bellomio A, Vincent PA, de Arcuri BF, Salomón RA, Morero RD, Farías RN (2004) The microcin J25 beta-hairpin region is important for antibiotic uptake but not for RNA polymerase and respiration inhibition. Biochem Biophys Res Commun 325(4):1454–1458. doi:10.1016/j.bbrc.2004.10.186
Bellomio A, Vincent PA, de Arcuri BF, Farías RN, Morero RD (2007) Microcin J25 has dual and independent mechanisms of action in Escherichia coli: RNA polymerase inhibition and increased superoxide production. J Bacteriol 189(11):4180–4186. doi:10.1128/jb.00206-07
Blond A, Cheminant M, Destoumieux-Garzón D, Ségalas-Milazzo I, Peduzzi J, Goulard C, Rebuffat S (2002) Thermolysin-linearized microcin J25 retains the structured core of the native macrocyclic peptide and displays antimicrobial activity. Eur J Biochem 269(24):6212–6222
Bollhagen R, Schmiedberger M, Barlos K, Grell E (1994) A new reagent for the cleavage of fully protected peptides synthesised on 2-chlorotrityl chloride resin. J Chem Soc Chem Commun 22:2559–2560. doi:10.1039/C39940002559
Braun V (1995) Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev 16(4):295–307
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta 1758(9):1184–1202. doi:10.1016/j.bbamem.2006.04.006
Choudhury HG, Tong Z, Mathavan I, Li Y, Iwata S, Zirah S, Rebuffat S, van Veen HW, Beis K (2014) Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state. Proc Natl Acad Sci USA 111(25):9145–9150. doi:10.1073/pnas.1320506111
Clarke DJ, Campopiano DJ (2007) Maturation of McjA precursor peptide into active microcin MccJ25. Org Biomol Chem 5(16):2564–2566
de Cristóbal RE, Solbiati JO, Zenoff AM, Vincent PA, Salomón RA, Yuzenkova J, Severinov K, Farías RN (2006) Microcin J25 uptake: His5 of the MccJ25 lariat ring is involved in interaction with the inner membrane MccJ25 transporter protein SbmA. J Bacteriol 188(9):3324–3328. doi:10.1128/jb.188.9.3324-3328.2006
Delgado MA, Rintoul MR, Farías RN, Salomón RA (2001) Escherichia coli RNA polymerase is the target of the cyclopeptide antibiotic microcin J25. J Bacteriol 183(15):4543–4550. doi:10.1128/jb.183.15.4543-4550.2001
Destoumieux-Garzón D, Duquesne S, Peduzzi J, Goulard C, Desmadril M, Letellier L, Rebuffat S, Boulanger P (2005) The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: role of the microcin Val11-Pro16 beta-hairpin region in the recognition mechanism. Biochem J 389(Pt 3):869–876. doi:10.1042/bj20042107
Ducasse R, Yan K-P, Goulard C, Blond A, Li Y, Lescop E, Guittet E, Rebuffat S, Zirah S (2012) Sequence determinants governing the topology and biological activity of a lasso peptide, microcin J25. ChemBioChem 13(3):371–380. doi:10.1002/cbic.201100702
Dupuy F, Morero R (2011) Microcin J25 membrane interaction: selectivity toward gel phase. Biochim Biophys Acta 1808(6):1764–1771. doi:10.1016/j.bbamem.2011.02.018
Dupuy FG, Chirou MV, de Arcuri BF, Minahk CJ, Morero RD (2009) Proton motive force dissipation precludes interaction of microcin J25 with RNA polymerase, but enhances reactive oxygen species overproduction. Biochim Biophys Acta 1790(10):1307–1313. doi:10.1016/j.bbagen.2009.07.006
Ferguson AL, Zhang S, Dikiy I, Panagiotopoulos AZ, Debenedetti PG, James Link A (2010) An experimental and computational investigation of spontaneous lasso formation in microcin J25. Biophys J 99(9):3056–3065. doi:10.1016/j.bpj.2010.08.073
Fields GB, Noble RL (1990) Solid-phase peptide-synthesis utilizing 9-fluorenylmethoxycarbonyl amino-acids. Int J Pept Protein Res 35(3):161–214
Gaussier H, Lefèvre T, Subirade M (2003) Binding of pediocin PA-1 with anionic lipid induces model membrane destabilization. Appl Environ Microbiol 69(11):6777–6784. doi:10.1128/AEM.69.11.6777-6784.2003
Hale JD, Hancock RE (2007) Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Exp Rev Anti-infect Ther 5(6):951–959. doi:10.1586/14787210.5.6.951
Hammami R, Zouhir A, Hamida JB, Neffati M, Vergoten G, Naghmouchi K, Fliss I (2009) Antimicrobial properties of aqueous extracts from three medicinal plants growing wild in arid regions of Tunisia. Pharm Biol 47(5):452–457. doi:10.1080/13880200902822604
Johnson NP, Mazarguil H, Lopez A (1996) Strandedness discrimination in peptide–polynucleotide complexes. J Biol Chem 271(33):19675–19679. doi:10.1074/jbc.271.33.19675
Kelly SM, Price NC (2000) The use of circular dichroism in the investigation of protein structure and function. Curr Protein Pept Sci 1(4):349–384
Liu S, Zhou L, Li J, Suresh A, Verma C, Foo YH, Yap EPH, Tan DTH, Beuerman RW (2008) Linear analogues of human β-defensin 3: concepts for design of antimicrobial peptides with reduced cytotoxicity to mammalian cells. ChemBioChem 9(6):964–973. doi:10.1002/cbic.200700560
Lopez FE, Vincent PA, Zenoff AM, Salomón RA, Farías RN (2007) Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J Antimicrob Chemother 59(4):676–680. doi:10.1093/jac/dkm009
Rintoul MR, de Arcuri BF, Salomón RA, Farı́as RN, Morero RD (2001) The antibacterial action of microcin J25: evidence for disruption of cytoplasmic membrane energization in Salmonella newport. FEMS Microbiol Lett 204(2):265–270. doi:10.1111/j.1574-6968.2001.tb10895.x
Mukhopadhyay J, Sineva E, Knight J, Levy RM, Ebright RH (2004) Antibacterial peptide microcin J25 inhibits transcription by binding within and obstructing the RNA polymerase secondary channel. Mol Cell 14(6):739–751. doi:10.1016/j.molcel.2004.06.010
Niklison Chirou M, Bellomio A, Dupuy F, Arcuri B, Minahk C, Morero R (2008) Microcin J25 induces the opening of the mitochondrial transition pore and cytochrome c release through superoxide generation. FEBS J 275(16):4088–4096. doi:10.1111/j.1742-4658.2008.06550.x
Pan SJ, Link AJ (2011) Sequence diversity in the lasso peptide framework: discovery of functional microcin J25 variants with multiple amino acid substitutions. J Am Chem Soc 133(13):5016–5023. doi:10.1021/ja1109634
Pan SJ, Cheung WL, Fung HK, Floudas CA, Link AJ (2011) Computational design of the lasso peptide antibiotic microcin J25. Protein Eng Des Sel 24(3):275–282. doi:10.1093/protein/gzq108
Patel G, Husman W, Jehanli AM, Deadman JJ, Green D, Kakkar VV, Brennand DM (1999) A cyclic peptide analogue of the loop III region of platelet-derived growth factor-BB is a synthetic antigen for the native protein. J Pept Res 53(1):68–74. doi:10.1111/j.1399-3011.1999.tb01618.x
Pavlova O, Mukhopadhyay J, Sineva E, Ebright RH, Severinov K (2008) Systematic structure–activity analysis of microcin J25. J Biol Chem 283(37):25589–25595. doi:10.1074/jbc.M803995200
Rosengren KJ, Clark RJ, Daly NL, Göransson U, Jones A, Craik DJ (2003) Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J Am Chem Soc 125(41):12464–12474. doi:10.1021/ja0367703
Sable S, Pons AM, Gendron-Gaillard S, Cottenceau G (2000) Antibacterial activity evaluation of microcin J25 against diarrheagenic Escherichia coli. Appl Environ Microbiol 66(10):4595–4597
Salomón RA, Farías RN (1993) The FhuA protein is involved in microcin 25 uptake. J Bacteriol 175(23):7741–7742
Salomón RA, Farías RN (1995) The peptide antibiotic microcin 25 is imported through the TonB pathway and the SbmA protein. J Bacteriol 177(11):3323–3325
Semenova E, Yuzenkova Y, Peduzzi J, Rebuffat S, Severinov K (2005) Structure–activity analysis of microcin J25: distinct parts of the threaded lasso molecule are responsible for interaction with bacterial RNA polymerase. J Bacteriol 187(11):3859–3863. doi:10.1128/jb.187.11.3859-3863.2005
Severcan F, Dorohoi DO (2008) FTIR studies of temperature influence on the DPPG model membrane. J Mol Struct 887(1–3):117–121. doi:10.1016/j.molstruc.2008.02.039
Solbiati JO, Ciaccio M, Farías RN, Salomón RA (1996) Genetic analysis of plasmid determinants for microcin J25 production and immunity. J Bacteriol 178(12):3661–3663
Soudy R, Wang L, Kaur K (2012) Synthetic peptides derived from the sequence of a lasso peptide microcin J25 show antibacterial activity. Bioorg Med Chem 20(5):1794–1800. doi:10.1016/j.bmc.2011.12.061
Sreerama N, Woody RW (2004) Computation and analysis of protein circular dichroism spectra. In: Ludwig B, Michael LJ (eds) Methods in enzymology, vol 383. Academic Press, St Louis, pp 318–351. doi:10.1016/S0076-6879(04)83013-1
Tam JP, Wu CR, Liu W, Zhang JW (1991) Disulfide bond formation in peptides by dimethyl-sulfoxide––scope and applications. J Am Chem Soc 113(17):6657–6662. doi:10.1021/Ja00017a044
Vincent PA, Delgado MA, Farías RN, Salomón RA (2004) Inhibition of Salmonella enterica serovars by microcin J25. FEMS Microbiol Lett 236(1):103–107. doi:10.1016/j.femsle.2004.05.027
Vincent PA, Bellomio A, de Arcuri BF, Farías RN, Morero RD (2005) MccJ25 C-terminal is involved in RNA-polymerase inhibition but not in respiration inhibition. Biochem Biophys Res Commun 331(2):549–551. doi:10.1016/j.bbrc.2005.03.220
Wilson K-A, Kalkum M, Ottesen J, Yuzenkova J, Chait BT, Landick R, Muir T, Severinov K, Darst SA (2003) Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J Am Chem Soc 125(41):12475–12483. doi:10.1021/ja036756q
Yan K-P, Li Y, Zirah S, Goulard C, Knappe TA, Marahiel MA, Rebuffat S (2012) Dissecting the maturation steps of the lasso peptide microcin J25 in vitro. ChemBioChem 13(7):1046–1052. doi:10.1002/cbic.201200016
Yuzenkova J, Delgado M, Nechaev S, Savalia D, Epshtein V, Artsimovitch I, Mooney RA, Landick R, Farias RN, Salomon R, Severinov K (2002) Mutations of bacterial RNA polymerase leading to resistance to microcin j25. J Biol Chem 277(52):50867–50875. doi:10.1074/jbc.M209425200
Acknowledgments
The authors express their gratefulness to Sophie Sablé for providing the E. coli strain harboring the plasmid pTUC202. François Bédard thanks the National Sciences and Engineering Research Council of Canada (NSERC) and the Fonds d’enseignement et de recherche de la Faculté de pharmacie de l’Université Laval for scholarships. The financial support of the Fonds de recherche du Québec-Nature et technologies (FQRNT) is gratefully acknowledged.
Conflict of interest
The authors declare that no personal relationship or interest had any influence on the design, execution, analysis or interpretation of the experiments reported herein.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hammami, R., Bédard, F., Gomaa, A. et al. Lasso-inspired peptides with distinct antibacterial mechanisms. Amino Acids 47, 417–428 (2015). https://doi.org/10.1007/s00726-014-1877-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1877-x