Summary
Background
Age-related macular degeneration is a chronic and progressive disease of the retina that occurs with increasing frequency with age, representing the leading cause of irreversible blindness in patients over 50 years of age. The loss of visual perception occurs due to neovascular proliferation. Antivascular endothelial growth factors revolutionized the treatment of exudative age-related macular degeneration.
Materials and methods
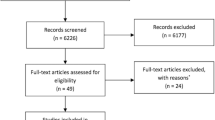
Our aim was to assess the systemic safety of intravitreal administration of ranibizumab, bevacizumab, or aflibercept in patients with neovascular age-related macular degeneration. The study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist. The primary outcomes included: all-cause death and all serious systemic adverse events. We calculated the incidence of systemic adverse events, relative risk of the outcomes, and 95% confidence intervals.
Results
This review included representative outcome data on 12,292 participants from 24 trials, which included both men and women aged 50 years or older with neovascular age-related macular degeneration. In bevacizumab-treated patients, the most frequent systemic adverse event in our analysis was nonocular hemorrhage. In ranibizumab-treated patients, systemic adverse events were not dose-dependent. The number of systemic adverse events was similar in patients treated with ranibizumab (4.73%) or with aflibercept (4.76%).
Conclusion
This meta-analysis did not find any statistically significant difference regarding the occurrence of death or other serious systemic adverse events such as treatment-emergent hypertension, myocardial infarction, stroke, or nonocular hemorrhage after intravitreal use of antivascular endothelial growth factors.
Zusammenfassung
Hintergrund
Die altersbedingte Makuladegeneration ist eine chronische und progressive Erkrankung der Netzhaut, die mit zunehmendem Alter gehäuft auftritt und die wichtigste Ursache für irreversible Blindheit bei Patienten über 50 Jahre darstellt. Zum Verlust der Sehfähigkeit bei der exsudativen Form der Erkrankung kommt es durch neovaskuläre Proliferation. Gegen vaskuläres endotheliales Wachstum wirkende Substanzen („anti-vascular endothelial growth factors“, Anti-VEGF-Faktoren) haben die Behandlung der exsudativen altersbedingte Makuladegeneration revolutioniert.
Material und Methoden
Das Ziel der vorliegenden Analyse war es, die systemische Sicherheit der intravitrealen Verabreichung von Ranibizumab, Bevacizumab und Aflibercept bei Patienten mit neovaskulärer altersbedingter Makuladegeneration zu untersuchen. Die Studie wurde nach der Checkliste „preferred reporting items for systematic reviews and meta-analyses“ (PRISMA) durchgeführt und dokumentiert. Primäre Zielvariablen waren Gesamtmortalität und alle schweren systemischen Nebenwirkungen. Es wurden die Inzidenz systemischer Nebenwirkungen, das relative Risiko und die 95%-Konfidenzintervalle berechnet. Die vorliegende Übersichtsarbeit enthält repräsentative Daten von 12.292 weiblichen und männlichen Teilnehmern über 50 Jahre, die an 24 Studien teilgenommen haben.
Ergebnisse
Zwischen der Inzidenz (4–5%) von systemischen Nebenwirkungen bei Ranibizumab (4,73%) und Aflibercept (4,76%) gab es keinen signifikanten Unterschied. Bei mit Bevacizumab behandelten Patienten bestand die häufigste systemische Nebenwirkung in der vorliegenden Studie in einer nichtokulären Blutung. Bei mit Ranibizumab behandelten Patienten wurde keine Dosisabhängigkeit hinsichtlich des Auftretens systemischer Nebenwirkungen festgestellt.
Schlussfolgerung
Die vorliegende Metaanalyse zeigt keinen signifikanten Unterschied in Bezug auf die Inzidenz von Todesfällen oder anderen schwerwiegenden Nebenwirkungen wie therapiebedingte Hypertension, Myokardinfarkt, Schlaganfall oder nichtokuläre Blutung nach intravitrealer Verabreichung verschiedener Anti-VEGF-Substanzen.



Similar content being viewed by others
References
Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98(5):629–38.
Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68(8):1029–36.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):5–11.
Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e16.
Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina (Philadelphia, Pa). 2006;26:859–70.
Ziemssen F, Sobolewska B, Deissler H, Deissler H. Safety of monoclonal antibodies and related therapeutic proteins for the treatment of neovascular macular degeneration: addressing outstanding issues. Expert Opin Drug Saf. 2016;15(1):75–87.
Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–24.
Semeraro F, Morescalchi F, Duse S, Gambicorti E, Cancarini A. Costagliola C pharmacokinetic and pharmacodynamic properties of anti-VEGF drugs after Intravitreal injection. Curr Drug Metab. 2015;16(7):572–84.
Tataru CP, Iancu R, Dogaroiu A, Diaconu C, Corbu C. Evaluation of the efficacy and safety of intravitreal bevacizumab for macular edema related to retinal vein occlusion. Farmacia. 2016;64(3):444–8.
Lee JH, Canny MD, De Erkenez A, Krilleke D, Ng YS, Shima DT, Pardi A, Jucker F. A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc Natl Acad Sci USA. 2005;102:18902–7.
Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121:936–45.
Karth PA, Feldman BH, Tripathy K, Shah VA. Bevacizumab. EyeWiki. San Francisco: American Academy of Ophthalmology; 2017.
Stewart MW. Aflibercept (VEGF-TRAP): the next anti-VEGF drug. Inflamm Allergy Drug Targets. 2011;10:497–508.
Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–8.
Csaky K, Do DV. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol. 2009;148:647–56.
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85.
Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56:95–113.
Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–93.
Ng EW, Shima DT, Calias P, Cunningham ET Jr., Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5(2):123–32.
Rinaldi M, Chiosi F, Dell’Omo R, Romano MR, Parmeggiani F, Semeraro F, Menzione M, Costagliola C. Intravitreal pegaptanib sodium (Macugen) for treatment of myopic choroidal neovascularization: a morphologic and functional study. Retina (Philadelphia, Pa). 2013;33(2):397–402.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31.
Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010;150(3):315–24.
Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, Rubio RG, Lai P, HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–56.
Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, Rubio RG, Lai P, HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181–92.
CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908.
Sharma S, Johnson D, Abouammoh M, Hollands S, Brissette A. Rate of serious adverse effects in a series of bevacizumab and ranibizumab injections. Can J Ophthalmol. 2012;47(3):275–9.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–98.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–411.
Schauwvlieghe AM, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MG, Peto T, Vingerling JR, Schlingemann RO. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. The BRAMD study. PLOS ONE. 2016;11(5):e153052.
Fischer C, Schäfer K, Dschietzig T, Hoerauf H. Analysis of cardiovascular diseases after the upload phase with intravitreal ranibizumab and bevacizumab in patients with exudative age-related macular degeneration. Ophthalmologe. 2016;113(7):589–95.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Kim JH, Lee DW, Chang YS, Kim JW, Kim CG. Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2101–9.
Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014; https://doi.org/10.1002/14651858.CD005139.pub3.
Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, D’Amico R, Dickersin K, Kodjikian L, Lindsley K, Loke Y, Maguire M, Martin DF, Mugelli A, Mühlbauer B, Püntmann I, Reeves B, Rogers C, Schmucker C, Subramanian ML, Virgili G. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014; https://doi.org/10.1002/14651858.CD011230.pub2.
Kodjikian L, Decullier E, Souied EH, Girmens JF, Durand EE, Chapuis FR, Huot L. Bevacizumab and ranibizumab for neovascular age-related macular degeneration: an updated meta-analysis of randomised clinical trials. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1529–37.
Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol. 2015;160(3):569–80.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
A. Popa Cherecheanu, R. Iancu, D. Vasile, R. Pirvulescu, A. Geamanu, C. Coman, and G. Iancu declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Popa-Cherecheanu, A., Iancu, R., Vasile, D. et al. Systemic side effects after intravitreal administration of antivascular endothelial growth factors for neovascular age-related macular degeneration. Spektrum Augenheilkd. 33, 110–116 (2019). https://doi.org/10.1007/s00717-017-0384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00717-017-0384-3