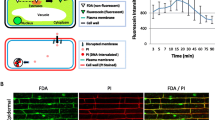
Abstract
Live imaging allows observations of cell structures and processes in real time, to monitor dynamic changes within living organisms compared to fixed organisms. Fluorescence microscopy was used to monitor the dynamic infection process of the nematode parasitic bacterium Pasteuria sp. and the sugarcane root-lesion nematode, Pratylenchus zeae. Under fluorescence microscopy, green-autofluorescent globules were observed in live control and Pasteuria sp.-infected nematodes. Only nematodes killed by Pasteuria sp. or heat treated displayed a diffuse pattern of autofluorescence. Propidium iodide (PI), used as a cell membrane integrity indicator, confirmed that the nematode’s cuticle acts as an impermeable barrier. PI stained cells/DNA of heat-treated control and Pasteuria sp.-infected P. zeae. PI as a counterstain facilitated the location of Pasteuria endospores on the cuticle surface of P. zeae. No PI staining was observed in sporangia and in endospores within the nematode body. However, PI specifically stained endospores on the cuticle surface and within the cuticle carcass showing, in mature propagules, a ring-like pattern. Live imaging, combined with fluorescence microscopy and fluorescent dyes such as PI, appears useful in live studies on plant nematode interactions with nematophagous bacteria.



Similar content being viewed by others
References
Anderson RV, Douglas GW, Ingham RE, Coleman DC (1979) A staining method for nematodes: Determination of nematode resistant stages and direct counts from soil. Trans Am Microsc Soc 98(2):213–218
Birchfield W, Antonopoulos AA (1976) Scanning electron microscopic observations of Duboscqia penetrans parasitizing root-knot larvae. J Nematol 8:272–273
Bird A (1986) The influence of the actinomycete, Pasteuria penetrans, on the host–parasite relationship of the plant-parasitic nematode, Meloidogyne javanica. Parasitology 93(3):571–580. https://doi.org/10.1017/S0031182000081270
Bird AF, Brisbane PG, McClure SG, Kimber RWL (1990) Studies in the properties of the spores of some populations of Pasteuria penetrans. J Invert Pathol 55:169–178
Carneiro RMDG, Randig O, Freitas LG, Dickson DW (1999) Attachment of endospores of Pasteuria penetrans to males and juveniles of Meloidogyne spp. Nematol 1(3):267–271. https://doi.org/10.1163/156854199508252
Chen ZX, Dickson DW (1998) Review of Pasteuria penetrans: biology, ecology and biological control potential. J Nematol 30:313–340
Ciancio A (2018) Biocontrol potential of Pasteuria spp. for the management of plant parasitic nematodes. CAB Reviews 13(013):1–13. https://doi.org/10.1079/PAVSNNR201813013
Forge TA, Macguidwin AE (1989) Nematode autofluorescence and its use as an indicator of viability. J Nematol 21(3):399–403
Giblin-Davis RM, Nong G, Preston JF, Williams DS, Center BJ, Brito JA, Dickson DW (2011) ‘Candidatus Pasteuria aldrichii’, an obligate endoparasite of the bacterivorous nematode Bursilla. Int J Syst Evol Microbiol 61:2073–2080. https://doi.org/10.1099/ijs.0.021287-0
Hudson B, Upholt WB, Devinny J, Vinograd J (1969) The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Nat Acad Sci USA 62:813–820. https://doi.org/10.1073/pnas.62.3.813
Jones KH, Kniss DA (1987) Propidium iodide as a nuclear counterstain for immunofluorescence studies on cells in culture. J Histochem Cytochem 35:123–125. https://doi.org/10.1177/35.1.2432112
Jones K, Kim DW, Park JS, Khang CH (2016) Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biology 16:69. https://doi.org/10.1186/s12870-016-0756-x
Joseph S, Gheysen G, Subramaniam K (2012) RNA interference in Pratylenchus coffeae: Knock down of Pc-pat-10 and Pc-unc-87 impedes migration. Mol Biochem Parasitol 186(1):51–59. https://doi.org/10.1016/j.molbiopara.2012.09.009
Md I, Md R, Md F, Hossain ATM, Sultana A (2016) Integrated management of seed borne nematode (Aphelenchoides besseyi) in T. Aman rice (Oryza sativa L.). The Agriculturists 13:79. https://doi.org/10.3329/agric.v13i1.26550
Mhatre PH, Eapen SJ, Chawla G, Pervez R, Agisha VN, Tadigiri S, Nagesh M (2020) Isolation and characterization of Pasteuria parasitizing root-knot nematode, Meloidogyne incognita, from black pepper fields in India. Egypt J Biol Pest Control 30:97. https://doi.org/10.1186/s41938-020-00296-z
Mohan S, Mauchline TH, Rowe J, Hirsch PR, Davies KG (2012) Pasteuria endospores from Heterodera cajani (Nematoda: Heteroderidae) exhibit inverted attachment and altered germination in cross-infection studies with Globodera pallida (Nematoda: Heteroderidae). FEMS Microbiol Ecol 79:675–684. https://doi.org/10.1111/j.1574-6941.2011.01249.x
Moody EH, Lownsbery BF, Ahmed JM (1973) Culture of the root-lesion nematode Pratylenchus vulnus on carrot disks. J Nematol 5:225–226
Oostendorp M, Hewlett TE, Dickson DW, Mitchell DJ (1991) Specific gravity of spores of Pasteuria penetrans and extraction of spore-filled nematodes from soil. J Nematol 23(4S):729–732
Overholt EP, Duffy MA, Meeks MP, Leach TH, Williamson CE (2020) Light exposure decreases infectivity of the Daphnia parasite Pasteuria ramose. J Plankton Res 42(1):41–44. https://doi.org/10.1093/plankt/fbz070
Öztürk L, Behmand T, Avcı GG, Bozbuğa R, Mirik M, Elekcioğlu IH (2020) Survey of Pasteuria, the parasitic bacterial group to plant parasitic nematodes in Turkey. Egypt J Biol Pest Control 30:64. https://doi.org/10.1186/s41938-020-00251-y
Perrine-Walker F, Stirling G, Guest D (2019) Pratylenchus zeae (Graham, 1951) Plant pathogen of the month - January 2019. APPS https://www.appsnet.org/Publications/potm/pdf/Jan19.pdf
Phani V, Rao U (2018) Revisiting the life-cycle of Pasteuria penetrans infecting Meloidogyne incognita under soil-less medium, and effect of streptomycin sulfate on its development. J Nematol 50(2):91–98. https://doi.org/10.21307/jofnem-2018-022
Ramouthar PV, Bhuiyan SA (2018) Nematodes parasites in sugarcane. In: Sikora RA, Coyne D, Hallmann J, Timper P (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CABI Publishing, Wallingford, pp 658–686
Rounds CM, Lubeck E, Hepler PK, Winship LJ (2011) Propidium iodide competes with Ca (2+) to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol 157:175–187. https://doi.org/10.1104/pp.111.182196
Santos DA, Dias-Arieira CR, Souto ER, Biela F, Cunha TPL, Rogério F, Silva TRB, Milani KF (2012) Reaction of sugarcane genotypes to Pratylenchus brachyurus and P. zeae. J Food Agric Environ 10:585–587
Sayre RM, Starr JF (1985) Pasteuria penetrans (ex Thorne, 1940) nom, rev., Comb.n., sp. n.a mycelia and endospore forming bacterium parasitic in plant parasitic nematodes. Proc Helminthol Soc Wash 52:149–165
Sayre RM, Starr MP, Golden A, Wergin WP, Endo BY (1988) Comparison of Pasteuria penetrans from Meloidogyne incognita with a related mycelia and endospore forming bacterial parasitic from Pratylenchus brachyurus. Proceedings of Helminthological Society of Washington 55:28–49
Sayre RM, Starr MP, Dickson DW, Preston JF, Giblin-Davis RM, Noel GR, Ebert D, Bird GW (2015) Pasteuria. In Bergey’s Manual of Systematics of Archaea and Bacteria (eds W. B. Whitman, F. Rainey, P. Kämpfer, M. Trujillo, J. Chun, P. DeVos, B. Hedlund and S. Dedysh). https://doi.org/10.1002/9781118960608.gbm00555
Serracin M, Schuerger A, Dickson D, Weingartner D (1997) Temperature-dependent development of Pasteuria penetrans in Meloidogyne arenaria. J Nematol 29:228–240
Stirling GR (1984) Biological control of Meloidogyne javanica with Bacillus penetrans. Phytopathol 74:55–60. https://doi.org/10.1094/Phyto-74-55
Stirling G (1985) Host specificity of Pasteuria penetrans within the genus Meloidogyne. Nematologica 31:203–209. https://doi.org/10.1163/187529285X00265
Stirling GR (2008) The impact of farming systems on soil biology and soilborne diseases: examples from the Australian sugar and vegetable industries — the case for better integration of sugarcane and vegetable production and implications for future research. Australas Plant Pathol 37:1–18. https://doi.org/10.1071/AP07084
Stirling GR (2014) Biological control of plant parasitic nematodes. 2nd edn. Soil ecosystem management in sustainable agriculture. CAB International, Wallingford
Stirling GR, Wong E, Bhuiyan S (2017) Pasteuria, a bacterial parasite of plant-parasitic nematodes: its occurrence in Australian sugarcane soils and its role as a biological control agent in naturally-infested soil. Australas Plant Pathol 46:563–569. https://doi.org/10.1007/s13313-017-0522-z
Sturhan D, Shutova TS, Akimov VN, Subbotin SA (2005) Occurrence, hosts, morphology, and molecular characterisation of Pasteuria bacteria parasitic in nematodes of the family Plectidae. J Invertebr Pathol 88(1):17–26. https://doi.org/10.1016/j.jip.2004.11.001
Sundararaj P, Mehta UK (1994) Influence of the lesion nematode, Pratylenchus zeae, on yield and quality characters of two cultivar of sugarcane. Nematologia Mediterranea 22:65–67
Suzuki T, Fujikura K, Higashiyama T, Takata K (1997) DNA Staining for fluorescence and laser confocal microscopy. J Histochem Cytochem 45(1):49–53. https://doi.org/10.1177/002215549704500107
Van Bezooijen J (2006) Methods and techniques for nematology. http://www.nematologia.com.br/files/tematicos/5.pdf
Wallace HR (1968) The dynamics of nematode movement. Annu Rev Phytopathol 6(1):91–114
Williams JR (1960) Studies on the nematode soil fauna of sugarcane fields in Mauritius. 5. Notes upon a parasite of root-knot nematodes. Nematologica 5:37–42
Williams JR (1967) Observations on parasitic protozoa in plant-parasitic and free-living nematodes. Nematologica 13:336–342
Williams AB, Stirling G, Hayward A, Perry J (1989) Properties and attempted culture of Pasteuria penetrans, a bacterial parasite of root-knot nematode (Meloidogyne javanica). J Appl Bacteriol 67:145–156. https://doi.org/10.1111/j.1365-2672.1989.tb03389.x
Acknowledgments
The authors would like to thank Prof. David Guest from the University of Sydney and Dr. Graham Stirling from Biological Crop Protection (Moggill, QLD 4070, Australia) for their support and use of laboratory materials as well as critical reading during manuscript preparation. The authors also thank Dr M.L. Walker for the careful reading of the manuscript.
Availability of data and material
Not applicable
Code availability
Not applicable
Funding
K.L. was supported by an Australian Awards Scholarships OASIS ID no: ST000HPH5.
Author information
Authors and Affiliations
Contributions
F.P.-W. designed and performed all live imaging experiments, acquired images and analyzed the micrographs, and wrote the manuscript. K.L. performed all Pasteuria-Pratylenchus infection experiments and P. zeae nematode carrot cultures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Handling Editor: Handling Editor: Georg Krohne
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
Fig.S1
a Light micrograph of filamentous rhizoid-like structures from a Pasteuria sp. germinating endospore germinating within P. zeae nematode (black arrowhead). b Close-up of (a) highlighting the filamentous structure (black arrowheads). c Mature female P. zeae infected with Pasteuria sp. endospores under UV fluorescence and light. Black arrowheads show globules with autofluorescence and the lack of autofluorescence colocalizing with Pasteuria sp. endospores (black arrows). c Example of PI staining the tail end of uninoculated P. zeae nematode (white arrowhead). d Uninoculated heat treated P. zeae stained with PI – note cells are PI stained inside the nematodes. e PI stained nematodes cells surrounding Pasteuria endospores in heat- treated Pasteuria sp. infected P. zeae nematode. Images in (b) and (c) were taken under WIB fluorescence only. Images in (c) and (e) were taken under WIB fluorescence and light to show the outline of nematodes. Scale bar is 5 μm in b, 10 μm in a and c, 20 μm in f, 50 μm in d and 100 μm in e. h head region, m median bulb, p pharynx, s stylet, t tail region (PNG 4472 kb)
Supplementary Video 1.
Live imaging of two live/motile control nematodes using the WIB2 filter under fluorescence showing autofluorescent globules. Light was used to show the outline of nematodes (20 frames/s) at x 400 magnification. (AVI 8732 kb)
Supplementary Video 2.
Live imaging of a live/motile Pasteuria sp.- infected nematode stained with PI using the WIB2 filter under fluorescence. Autofluorescent globules – green fluorescence; Pasteuria sp. endospores on the cuticle of a live nematode- red fluorescence; Anterior - head region; Posterior – tail end (20 frames/s) at x 400 magnification. (AVI 5582 kb)
Rights and permissions
About this article
Cite this article
Perrine-Walker, F., Le, K. Propidium iodide enabled live imaging of Pasteuria sp.-Pratylenchus zeae infection studies under fluorescence microscopy. Protoplasma 258, 279–287 (2021). https://doi.org/10.1007/s00709-020-01567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01567-0