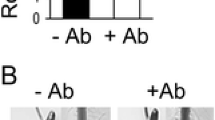
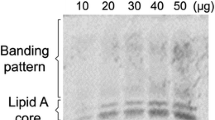
Abstract
Azospirillum is a plant growth-promoting rhizobacteria (PGPR) able to enhance the growth of wheat. The aim of this study was to test the effect of Azospirillum brasilense cell wall components on superoxide (O2·−) production in wheat roots and the effect of oxidative stress on A. brasilense viability. We found that inoculation with A. brasilense reduced O2·− levels by approx. 30 % in wheat roots. Inoculation of wheat with papain-treated A. brasilense, a Cys protease, notably increased O2·− production in all root tissues, as was observed by the nitro blue tetrazolium (NBT) reduction. However, a 24-h treatment with rhizobacteria lipopolysaccharides (50 and 100 μg/mL) alone did not affect the pattern of O2·− production. Analysis of the effect of plant cell wall components on A. brasilense oxidative enzyme activity showed no changes in catalase activity but a decrease in superoxide dismutase activity in response to polygalacturonic acid treatment. Furthermore, A. brasilense growth was only affected by high concentrations of H2O2 or paraquat, but not by sodium nitroprusside. Our results suggest that rhizobacterial cell wall components play an important role in controlling plant cell responses and developing tolerance of A. brasilense to oxidative stress produced by the plant.





Similar content being viewed by others
References
Abel S, Theologis A (2010) Odyssey of auxin. Cold Spring Harb Perspect Biol 10:a004572
Arthikala M-K, Sanchez-Lopez R, Nava N, Santana O, Cardenas L, Quinto C (2014) RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol 202:886–900
Bakker PAHM, Pieterse CMJ, Van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97:239–243
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bestwick CS, Adam AL, Puri N, Mansfield JW (2001) Characterization of and changes to pro- and anti-oxidant enzyme activities during the hypersensitive reaction in lettuce (Lactuca satia L.). Plant Sci 161:497–506
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Camilios-Neto D, Bonato P, Wassem R, Tadra-Sfeir MZ, Brusamarello-Santos LCC, Valdameri G, Donatti L, Faoro H, Weiss VA, Chubatsu LS, Pedrosa FO, Souza EM (2014) Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genomics 15:378
Carol RJ, Dolan L (2006) The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot 57:1829–1834
Causin HF, Roqueiro G, Petrillo E, Láinez V, Pena LB, Marchetti CF, Gallego SM, Maldonado SI (2012) The control of root growth by reactive oxygen species in Salix nigra Marsh. Seedlings. Plant Sci 183:197–205
Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ et al (2006) Priming: getting ready for battle. Mol Plant-Microbe Interact 19:1062–1071
Darveau PR, Hancock REW (1983) Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol 155:831–838
Delgado M, Yero D, Niebla O, González S, Climent Y, Pérez Y, Cobas K, Caballero E, García D, Pajón R (2007) Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine 25:8420–8431
Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Drogue B, Sanguin H, Borland S, Prigent-Combaret C, Wisniewski-Dye F (2014) Genome wide profiling of Azospirillum lipoferum 4B gene expression during interaction with rice roots. FEMS Microbiol Ecol 87:543–555
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Fischer SE, Marioli JM, Mori GB (2003) Effect of root exudates on the exopolysaccharide composition and the lipopolysaccharide profile of Azospirillum brasilense Cd under saline stress. FEMS Microbiol Lett 219:153–162
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863
Furlong CE (1987) Osmotic-shock-sensitive transport systems. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol 1. American Society for Microbiology, Washington, D.C., pp 768–796
Godlewska R, Wisniewska K, Pietras Z, Jagusztyn-Krynicka EK (2009) Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol Lett 298:1–11
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signalling processes. Soil Biol Biochem 37:395–412
Hérouart D, Sigaud S, Moreau S, Frendo P, Touati D, Puppo A (1996) Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J Bacteriol 178:6802–6809
Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21:829–837
Kim YC, Miller CD, Anderson AJ (2000) Superoxide dismutase activity in Pseudomonas putida affects utilization of sugars and growth on root surfaces. Appl Environ Microbiol 66:1460–1467
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2 •−, H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth promoting rhizobacteria. Anton Leeuw 86:1–25
Menard R, Khouri HE, Plouffe C, Dupras R, Ripoll D, Vernet T, Tessier DC, Lalberte F, Thomas DY, Storer AC (1990) A protein engineering study of the role of aspartate 158 in the catalytic mechanism of papain. Biochemistry 29:6706–6713
Nanda AK, Andrio E, Marino D, Pauly N, Dunand C (2010) Reactive oxygen species during plant-microorganism early interactions. J Integr Plant Biol 52:195–204
Neufert C, Pai RK, Noss EH, Berger M, Boom WH, Harding CV (2001) Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol 167:1542–1549
Newman MA, Sundelin T, Nielsen JT, Gitte Erbs G (2013) MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front Plant Sci 4:1–14
Nurnberger T, Brunner F, Kemmerling B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198:249–266
Pauly N, Pucciariello C, Mandon K, Innocenti G, Jamet A, Baudouin E, Herouart D, Frendo P, Puppo A (2006) Reactive oxygen and nitrogen species and glutathione: key players in the legume-Rhizobium symbiosis. J Exp Bot 57:1769–1776
Porcella SF, Schwan TG (2001) Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms. J Clin Invest 107:651–656
Rau H, Revets H, Cornelis P, Titzmann A, Ruggli N, McCullough KC, Summerfield A (2006) Efficacy and functionality of lipoprotein OprI from Pseudomonas aeruginosa as adjuvant for a subunit vaccine against classical swine fever. Vaccine 24:4757–4768
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28:679–688
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125:1591–1602
Sha J, Fadl AA, Klimpel GR, Niesel DW, Popov VL, Chopra AK (2004) The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect Immun 72:3987–4003
Sha J, Agar SL, Baze WB, Olano JP, Fadl AA, Erova TE, Wang S, Foltz SM, Suarez G, Motin VL, Chauhan S, Klimpel GR, Peterson JW, Chopra AK (2008) Braun lipoprotein (Lpp) contributes to virulence of yersiniae: potential role of Lpp in inducing bubonic and pneumonic plague. Infect Immun 76:1390–1409
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201:850–861
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49:16–26
Tsuge H, Nishimura T, Tada Y, Asao T, Turk D, Turk V, Katunuma N (1999) Inhibition mechanism of cathepsin L-specific inhibitors based on the crystal structure of papain–CLIK148 complex. Biochem Biophys Res Commun 266:411–416
Tsukagoshi H (2012) Defective root growth triggered by oxidative stress is controlled through the expression of cell cycle-related genes. Plant Sci 197:30–39
Uhlig H (1998) Industrial enzymes and their applications. Wiley, New York
Van Loon LC (2007) Plant response to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Van Loon LC, Glick BR (2004) Increased plant fitness by rhizobacteria. In: Sandermann H (ed) Molecular ecotoxicology of plants. Ecological Suites. Springer, Berlin, pp 178–205
Verhagen BWM, Glazebrook J, Zhu T, Chang HS, van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant-Microbe Interact 17:895–908
Wang WL, Chen N, Wu H, Zang H, Gao S, Yang Y, Xie S, Gao X (2012) Comparative proteomic analysis of rice seedlings in response to inoculation with Bacillus cereus. Lett Appl Microbiol 56:208–215
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318
Acknowledgments
This study was supported by the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo, México.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Adrienne R. Hardham
Rights and permissions
About this article
Cite this article
Méndez-Gómez, M., Castro-Mercado, E., Alexandre, G. et al. Oxidative and antioxidative responses in the wheat-Azospirillum brasilense interaction. Protoplasma 253, 477–486 (2016). https://doi.org/10.1007/s00709-015-0826-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0826-1