Abstract
This paper presents the development of spectrofluorimetric and smartphone-based detection methods for gentamicin determination using fluorescamine as a reagent. The research included selecting excitation (415 nm) and emission (489 nm) wavelengths, reaction time, and conditions like reagent concentration, and pH of the reaction medium. Moreover, the optimal operating parameters of the smartphone camera, like ISO, white balance, camera shutter, and RGB model channel were selected. Analytical parameters of the developed spectrofluorimetric and smartphone-based methods were estimated including the linear range: 0.04–15.00 mg dm−3 and 0.18–1.20 mg dm−3, respectively, limits of detection and quantification: 0.01 and 0.04 mg dm−3, and 0.06 and 0.18 mg dm−3, respectively, and precision (CV, n = 6): 5.2% and 2.8%, respectively. The proposed approaches were successfully applied to determine gentamicin in pharmaceutical samples. The obtained results were consistent with values declared by manufacturers and satisfactory recovery values, 93.2–113.6% were obtained for both spectrofluorimetric and smartphone-based methods. The developed fluorimetric method with smartphone-based detection provides a low limit of detection specific to spectrofluorimetric methods whereas the measurement system is a simple, easily accessible, compact, and low-cost device. Hence, it can become a competitive alternative to other gentamicin determination methods.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gentamicin is one of the aminoglycoside antibiotics used to treat serious infections with Gram-negative bacteria, and also some Gram-positive bacteria [1]. The bacterium Micromonospora purpurea can naturally produce gentamicin, and the first reports of gentamicin being used for therapeutic purposes were patented in the 1960s [2]. Gentamicin is most commonly found in eye drops at a concentration of 3 mg cm−3, in ointments it is usually 1 mg g−1. In solutions for injection, its concentration is about 40 mg cm−3, but such solutions are diluted before use. Gentamicin has shown antibacterial activity in eye infections, urinary tract diseases, respiratory problems, and surgical procedures [3]. Also, the World Health Organization suggests the use of ampicillin and gentamicin as first-line drugs to treat sepsis symptoms, especially in Third World countries [4].
Since the introduction of gentamicin in the pharmaceutical industry, various methods have been developed to determine its content in pharmaceutical and biological samples. Among them high-performance liquid chromatography (HPLC) with different types of detection [5,6,7,8], mass spectrometry [9], capillary electrophoresis [10], enzyme-linked immunosorbent assay (ELISA) [11], electrochemical method and sensor [12, 13]. The literature also describes the use of spectrophotometric [14, 15], spectrofluorimetric [16, 17], and chemiluminescent [18] methods for the determination of this analyte. Chromatographic methods have the advantage of being able to separate mixtures simultaneously. However, the instruments’ rather high cost and low portability may pose some limitations to application. On the other hand, spectrometric methods developed for the determination of aminoglycoside antibiotics benefit from less expensive and more readily available equipment.
Over the past 10 years, interest in using a digital imaging system for chemical analysis has grown significantly. A digital smartphone imaging system is often used for this purpose [19]. Smartphone-based detection was adapted to photometric methods [20,21,22] and increasingly to fluorimetric methods [23,24,25]. Smartphones as detection systems have been used for the analysis of biological samples [26, 27], food [28, 29], or environmental samples [30, 31]. Point-of-care photometric methods and assays have also been developed for the determination of antibiotics from the aminoglycoside group–streptomycin [32] and kanamycin [33]. The digital imaging system uses, inter alia, a smartphone camera, so it is possible to conduct a fairly quick analysis in situ. The analytical signal in a digital imaging system is represented by a value extracted from one of the channels, which is the colour component in the chosen colour representation (CMYK, HSL, HSV, RGB, among others). The use of the RGB colour representation is preferred in smartphone-based detection studies due to its simplicity. In RGB analysis, colour is decomposed and quantified into red (R), green (G), and blue (B) channels. Various software is used to decompose and obtain numerical values for a particular channel, of which one of the most popular image analysis software for the RGB model is ImageJ. This application is installed on a computer and, once the images are transferred to the computer, it is possible to acquire values for the R, G, and B channels [34, 35].
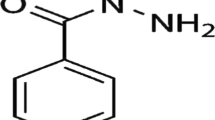
As there is an opportunity to adapt fluorimetric methods in analytical systems with smartphone-based detection, the present study aimed to develop a novel spectrofluorimetric method for determining gentamicin and adapt it in a system with smartphone-based detection. The study used the reaction of gentamicin with fluorescamine. This fluorophore has been previously reported in the literature for the determination of another antibiotic from the aminoglycoside group, tobramycin [36]. However, to the authors’ knowledge, a spectrofluorimetric method for the determination of gentamicin based on the reaction with fluorescamine and its adaptation to a smartphone-based digital imaging detection device has not yet been reported. In this study, excitation and emission wavelengths, reaction time, as well as reaction conditions and conditions for smartphone-based detection measurements were established. Validation of spectrofluorimetric and smartphone-based methods was performed. The methods were applied to the determination of gentamicin in drug samples. The developed approach with digital imaging detection using a smartphone as a portable measuring device offers the possibility of increasing the availability of the developed method while reducing equipment costs and maintaining the good sensitivity of the fluorimetric-based method.
Results and discussion
Preliminary studies
The research was started by testing the feasibility of spectrofluorimetric determination of gentamicin based on reaction with fluorescamine. Preliminary studies were conducted under the conditions developed for the method for the determination of tobramycin (also an aminoglycoside antibiotic) using fluorescamine [36]. According to this method, the reaction was conducted in a borate buffer medium of pH = 8.5, with fluorescamine concentration of 0.12 g dm−3, and a reaction time of 5 min. The excitation (λex = 415 nm) and emission (λem = 489 nm) wavelengths for the compound of gentamicin with fluorescamine were established based on spectrofluorimetric measurements of 3D spectrum. Moreover, at the selected excitation wavelength, appropriate fluorescent spectra for the compound of gentamicin with fluorescamine were measured in a concentration range of analyte from 0.00 to 4.00 mg dm−3, with fluorescamine concentration of 0.12 g dm−3 in borate buffer medium of pH 8.5. The obtained 3D spectrum, the excitation, and emission spectra obtained based on the 3D spectrum (both for a gentamicin concentration of 300 mg dm−3), and fluorescence spectra are shown in Fig. 1a–c, respectively.
The obtained 3D spectrum (a), the excitation and emission spectra obtained based on the 3D spectrum (b), both for gentamicin concentration 300 mg dm−3, and fluorescence spectra for different concentrations of gentamicin (c): 0.00 mg dm−3 (from below: blue line); 0.60 mg dm−3 (orange line), 0.80 mg dm−3 (grey line); 1.00 mg dm−3 (yellow line); 1.20 mg dm−3 (light blue line); 2.00 mg dm−3 (green line); 3.00 mg dm−3 (dark blue line); 4.00 mg dm−3 (red line); fluorescamine concentration 0.12 g dm−3, borate buffer, pH = 8.5; measurements taken after 5 min (color figure online)
In the study, it was also decided to select the optimal reaction time of gentamicin with fluorescamine. For this purpose, the change in fluorescence intensity over time was measured for a gentamicin concentration of 5.00 mg dm−3 under the conditions described above. The results, presented in Online Resource 1 (Supplementary Material), show that the maximum value of fluorescence intensity is reached approximately 3 min after the beginning of the reaction between gentamicin and fluorescamine. Therefore, the next studies for both spectrofluorimetric and smartphone digital imaging detection were performed for a reaction time of 3 min.
Selection of optimal conditions for smartphone-based detection system
Measurements with the smartphone were conducted in a 3D printed holder based on a previously developed device [37]. A scheme of this holder is shown in Fig. 5 in the “Experimental” section and a picture of the entire measurement system is available in Online Resource 2 (Supplementary Material). The next part of the study concerned the selection of the RGB model channel, optimal camera settings, and reaction conditions for the smartphone-based detection system.
An important part of the smartphone-based study was the selection of an appropriate channel, whose values represented the analytical signal in subsequent studies. For this purpose, gentamicin solutions were prepared in the range of 0.10–1.40 mg dm−3 at a fluorescamine concentration of 0.12 g dm−3. One solution containing gentamicin at a given concentration was prepared. Five images were taken for each solution. Error bars for images taken for a given solution are not shown in the graphs due to the small standard deviation values (smaller than the size of the data point). The obtained calibration graphs (for all three channels R, G, and B) are presented in Fig. 2a. Analysing the parameters of the obtained graphs, it has been decided that the optimal channel for collecting the analytical signal was the G (green) channel, for which the highest sensitivity and the best linear fitting of the calibration graph were achieved (y = 53.22x − 1.34, R2 = 0.993).
Calibration graphs; a for three channels with smartphone-based detection: R (from below: red circle), y = −4.32x + 6.31, R2 = 0.744; G (green circle) y = 53.22x − 1.34, R2 = 0.993; B (blue circle) y = 40.90x + 101.31, R2 = 0.858; b for different parameters of ISO with smartphone-based detection: ISO 100 (from below: green circle) y = 64.23x + 0.54, R2 = 0.993; ISO 200 (grey circle) y = 130.97x + 6.17; ISO 400 (black circle) y = 223.20x + 7.87; ISO 600 (blue circle) y = 269x + 12.95; ISO 800 (yellow circle) y = 437.21x + 11.79; signals based on measurements for a single gentamicin solution (color figure online)
As the ISO parameter determines the sensitivity of the camera’s sensor to the light, in the next part of the study, the optimal ISO parameter was selected from among values of 100, 200, 400, 600, and 800. Measurements were performed for the standards of gentamicin in concentration from 0.00 to 1.40 mg dm−3 and fluorescamine 0.12 g dm−3. The obtained calibration graphs are shown in Fig. 2b.
Analysing the obtained results, it can be noticed that for ISO of 100 the least sensitivity was obtained but the widest linear range with the possibility of practical application for gentamicin determination, and this value was selected for further studies.
In the next step of the research, the shutter speed and white balance were selected. The test was conducted similarly to the previous ones, under the selected measurement conditions. Measurements were performed successively for different smartphone camera shutter speeds of 1/25, 1/30, 1/35, and 1/40, in each case for a series of gentamicin solutions. The 1/30 shutter was selected for further testing, at which better calibration graph fitting than for 1/25 shutter speed and simultaneously better sensitivity (y = 47.75x + 6.88, R2 = 0.993, Fig. 3a) than for 1/35 and 1/40 shutter speeds were obtained.
Study of the influence of camera shutter (a) and white balance (b) on the parameters of calibration graphs; a camera shutter: 1/25 (from above: yellow circle) y = 50.69x + 10.04, R2 = 0.986, 1/30 (green circle) y = 47.75x + 6.88, R2 = 0.993, 1/35 (blue circle) y = 43.65x + 6.57, R2 = 0.994, 1/40 (black circle) y = 38.53x + 6.30, R2 = 0.998; b white balance: fluorescent (from above: green circle) y = 40.96x − 1.95, R2 = 0.988, cloudy (yellow circle) y = 41.11x − 3.47, R2 = 0.973, auto (blue circle) y = 38.54x − 4.69, R2 = 0.994, daylight (black circle) y = 37.53x − 6.92, R2 = 0.977, incandescent (grey circle) y = 36.33x − 6.88, R2 = 0.981; signals based on measurements for a single gentamicin solution (color figure online)
The influence of white balance was also studied. White balances such as cloudy, daylight, fluorescent, auto, and incandescent were analysed. It turned out that white balance did not have a significant effect on the slope of the calibration graph (Fig. 3b), and fluorescent white balance was selected for further research.
Selection of reaction conditions for smartphone-based detection
In the studies performed with the use of a smartphone-based detection, it was also decided to test the optimal concentration of fluorescamine and pH of the reaction medium (borate buffer).
To this aim, for fluorescamine concentrations of 0.06, 0.08, and 0.12 g dm−3, calibration graphs were prepared with the gentamicin concentration range of 0.20–1.40 mg dm−3 at pH 8.5 (borate buffer). The best parameters of the calibration graph were obtained using a fluorescamine concentration of 0.12 g dm−3, hence this concentration was selected for further research.
Subsequently, the selection of the optimal pH medium for the reaction of gentamicin and fluorescamine was performed. For this purpose, the pH of borate buffer 8.0, 8.5, 9.0, and 9.5 was tested in each case for a range of gentamicin concentrations of 0.00–1.20 mg dm−3. The optimal borate buffer pH was found to be pH = 9.5, as the calibration graph for this pH showed the best sensitivity and linear fitting (y = 48.67x + 5.26, R2 = 0.995) (Online Resource 3, Supplementary Material). A borate buffer of pH 9.5 was used for further studies.
The parameters selected in the optimisation process are summarised in Table 1. In subsequent studies, the established reaction conditions were also used for spectrofluorimetric measurements.
Method verification
Using the selected reaction conditions and camera settings (Table 1), the calibration graphs were prepared. Based on calibration graphs, the linearity for gentamicin determination was established in the range of 0.20–1.20 mg dm−3 for the smartphone-based detection method and in the whole studied concentration range of 0.10–15.00 mg dm−3 for the spectrofluorimetric method. Three solutions each containing gentamicin at a given concentration were prepared. The obtained calibration graphs are shown in Fig. 4.
Calibration graphs for a fluorimetric method with a smartphone-based detection system, (green circle), y = 52.32x + 2.09, R2 = 0.996; and b spectrofluorimetric method: in the range of 0.10–1.00 and 0.10–15.00 mg dm−3, (blue circle), y = 208.11 + 7.79, R2 = 0.999; bars indicate the standard deviation determined based on signals for three independent gentamicin solutions (color figure online)
In the selected conditions, the developed smartphone-based detection and spectrofluorimetric methods were applied to determine gentamicin at concentrations of 0.20, 0.30, 0.40, 0.50, 0.60, 0.70, and 0.80 mg dm−3 in model samples under the same experimental conditions. Solutions of model samples were prepared according to the “Samples” section. Analytical signals recorded for gentamicin model samples were not part of the calibration graph. The results obtained for both methods are compiled in Table 2 with calculated values of relative error (RE) and coefficient of variation (CV, n = 3).
The same set of solutions was used for analysis using smartphone-based detection and spectrofluorimetric detection hence the differences in the results are due to the method of detection. Comparing the results of gentamicin determination in model samples using smartphone-based detection and spectrofluorimetric methods, it can be concluded that results consistent with each other were obtained. The differences seen in Table 2 in the ǀREǀ values determined for the same concentration values (e.g., for the determined concentration of 0.41 mg dm−3, the ǀREǀ values are 2.9 and 1.4% for different methods) are because the relative error values were calculated using more decimal places than for the concentrations shown in Table 2. Analysing achieved analytical parameters of the smartphone-based detection method, it can be noticed that the proposed approach features good accuracy (RE < 8.1%, only in one case higher than 2.9%) and precision (CV < 9.2%). For the spectrofluorimetric determination of gentamicin, the results also show good precision (CV < 8.1%, only in one case higher than 5.3%) and very good accuracy (RE < 3.6%).
Analytical parameters
Analytical parameters such as limit of detection (LOD), limit of quantification (LOQ), linear range, and coefficient of variation (CV) were determined for both, the smartphone-based and spectrofluorimetric methods. The obtained parameters are summarised in Table 3. The limit of detection (LOD) was established as a 3.3 times standard deviation of results obtained for the concentration of gentamicin 0.10 mg dm−3 divided by the calibration graph slope value. The limit of quantification was estimated as 3 times the LOD.
It should be noted that for the smartphone-based detection system, LOD is 0.06 mg dm−3 and LOQ is 0.18 mg dm−3 whereas, for the spectrofluorimetric method, lower values of LOD and LOQ were obtained, 0.01 and 0.04 mg dm−3, respectively. A linear range for smartphone-based detection was between 0.18 and 1.20 mg dm−3, while for the spectrofluorimetric method, it was much wider (0.04–15.00 mg dm−3). Nevertheless, for the analysis of drugs, the linear range obtained for smartphone-based detection is sufficient. The precision values (n = 6) obtained for both methods are comparable and satisfactory (1.7–2.8% for smartphone-based method and 4.1–5.2% for spectrofluorimetry). Analysing the analytical parameter values for both methods, it can be concluded that the results obtained for smartphone-based detection are very close to those obtained spectrofluorimetrically. The developed fluorimetric method based on the use of the smartphone-based detection device has the potential to be successfully applied to the analysis of authentic samples and become competitive with the traditional spectrofluorimetric method.
Determination of gentamicin in pharmaceutical samples
The proposed approach for gentamicin determination with smartphone-based detection was used to determine this antibiotic in three drug samples of eye drops. Sample solutions were prepared according to the “Analytical procedure” section. In addition, recovery studies were carried out to check the possible influence of the sample matrix on the result. For the study, the samples were diluted 1000 times so the effect of the sample matrix on the signal was limited, however, Drug 3 contained another active ingredient that could affect the signal, so it was decided to perform recovery studies. Drug samples were spiked with the standard before the whole procedure. Obtained results with estimated analytical parameters (RE–calculated concerning values declared by a manufacturer, CV, and recovery, R) for smartphone-based detection and spectrofluorimetric detection are summarised in Table 4.
Analysing the obtained results, it can be concluded that the determined values for both methods are consistent with each other and in agreement with those declared by the manufacturers. The recovery results obtained confirm that the sample matrix did not affect the determination results for Drugs 1 and 2. In the case of Drug 3, the most different but acceptable recovery results were obtained, which confirmed the applicability of the method for Drug 3 as well. The results for both methods were obtained with good precision, not exceeding 7.1%. Thus, it can be concluded that the method based on the use of a smartphone-based detection system has the potential to be successfully applied for the determination of this antibiotic in medicine samples as an alternative to the spectrofluorimetric method.
Table 5 summarises selected analytical parameters of the methods available in the literature for the determination of gentamicin.
Analysing the LOD parameters shown in Table 5, it can be seen that the proposed method has a very competitive detection limit, only five reported methods, LC with fluorescence detection [7], ESI MS [9], ELISA [11], and other spectrofluorimetric methods [16, 17] offered lower detection limits.
It should be noted that in fluorimetric methods, the irradiance significantly affects the sensitivity and detection limit. It is therefore important to maintain constant sample illumination conditions during measurements. Lower limits of quantification could be achieved in the device by designing locations for the installation of e.g. four diodes whose radiation would be directed at the sample. Also, the use of lenses that collimate and focus the light in the image area would be also helpful to locally increase the intensity of the radiation in the image area and consequently the sensitivity of the determination.
Considering the linear range, the spectrofluorimetric method offers a fairly competitive range, in the range of two orders of magnitude, while the smartphone-based detection method achieved narrower linearity in the range of about one order of magnitude. This may represent a certain limitation of the method but does not prevent its practical application. Comparing the precision values, the methods have an acceptable precision, comparable to other methods reported in the literature. Hence, it can be concluded that the method has the potential to find analytical applications.
Based on the literature, it should be noted that the determination of gentamicin by fluorescamine using a smartphone-based detection system and a spectrofluorimetric method has not yet been reported. Particularly noteworthy is the developed fluorimetric method based on the use of smartphone-based detection. The method retains the low limit of detection characteristic for spectrofluorimetric methods and offers reliable analytical results while being simple and was implemented using a readily available, compact, and low-cost device. It can provide an alternative simple system for gentamicin determination.
The research conducted was done using a single smartphone. Smartphones differ in camera resolution and quality, as well as operating system (Android, iOS). Undoubtedly, further studies could include checking the analytical parameters of the method using different smartphones. In the literature, it is difficult to find fluorimetric methods for which determination results obtained with several smartphones have been compared, while good repeatability has been observed in photometric measurements [22]. However, it is worth noting that the proposed approach uses an application that includes a selection of the same parameters available for most smartphones (ISO parameter, white balance, shutter speed), which greatly simplifies the process of adapting the proposed method using a different smartphone.
There are a number of spectrophotometric methods in the literature that are adapted to smartphone-based detection, a trend that is in line with the principles of green analytical chemistry. The adaptation of spectrofluorimetric methods to smartphone-based detection is even more attractive because of their analytical performance, especially the low detection limits. A practical advantage of these devices is their compactness, low cost, and easy accessibility, which increases the availability of analytical methods and the possibility of implementing them under non-laboratory conditions. This also creates new opportunities for the development of spectrofluorimetric methods.
Conclusion
A novel spectrofluorimetric method for the determination of gentamicin based on the reaction of gentamicin with fluorescamine was developed and adapted to a smartphone-based detection system. Both approaches, spectrofluorimetric and smartphone-based detection offer the possibility of obtaining reliable analytical results. However, smartphone-based detection is characterized by a narrower linear range and a higher limit of quantification than the spectrofluorimetric method. The spectrofluorimetric and smartphone-based detection methods were successfully applied to the determination of gentamicin in pharmaceutical samples. The developed smartphone-based approach offers the possibility of increasing the availability of the spectrofluorimetric method while reducing instrument costs and approaching the sensitivity of the spectrofluorimetric method. The LOD of the developed method is low enough to allow, for example, the determination of gentamicin in samples from treated patients. However, additional research is needed to adapt the method to the analysis of biological samples. In this respect, the method can be considered as an intermediate step on the way to inexpensive Point-of-Care analysis.
Experimental
The following reagents were used to prepare the solutions: acetone (≥ 99.5%, Chempur, Piekary Śląskie, Poland), 0.1 mol dm−3 sodium hydroxide (Chempur, Piekary Śląskie, Poland), 10 mg cm−3 gentamicin solution (Sigma-Aldrich, St. Louis, USA), boric acid (Avantor Performance Materials Poland S.A., Gliwice, Poland), potassium chloride (Chempur, Piekary Śląskie, Poland), fluorescamine (≥ 98%, Pol-Aura, Dywity, Poland). Fluorescamine solutions of 0.75, 1.0, and 1.5 g dm−3 in acetone were prepared. Borate buffers (pH: 8.0, 8.5, 9.0, 9.5) were prepared as follows: 0.620 g boric acid and 0.750 g potassium chloride were dissolved in 100 cm3 water. The pH for each buffer was established with 0.1 mol dm−3 sodium hydroxide solution, then solutions were made up to the volume of 200 cm3 with deionised water. Buffers were filtered before measurements using a 0.45 µm pore Whatman syringe filter. The gentamicin solutions used for the measurements were prepared by the appropriate dilution of a gentamicin solution with a concentration of 10 mg cm−3 (Sigma-Aldrich, St. Louis, USA) in deionised water. During the study, deionised water (0.05 μS cm−1, Sprint 20UV System, Hydrolab, Poland) was used to prepare solutions.
Samples
The proposed method was verified based on the determination of gentamicin in model samples—gentamicin solution (Sigma-Aldrich, St. Louis, USA) of the different known concentrations. Furthermore, samples of drugs were analysed: two different samples of Drug 1 and Drug 2, 3 mg cm−3 (Poland), and one of Drug 3, 5 mg cm−3, (Germany). Solutions of model and drug samples were prepared according to the procedure described in the “Analytical procedure” section. For the recovery studies, samples were spiked with 3 mg cm−3 of gentamicin.
Instrumentation
Spectrofluorimetric measurements were performed using Spectro Fluorophotometer RF-6000 (Shimadzu, Kioto, Japan) with LabSolutions RF software (Shimadzu, Kioto, Japan).
The smartphone equipped with Open Camera® software (by Mark Harman, Google Play) and a 3D printed holder with an LEDs system, designed based on Granica paper [37] enabled the use of smartphone-based detection system for fluorimetric measurements. The holder was designed (IronCAD, Santa Clara, USA) and printed using Stereolithography (SLA) 3D printer (Formlabs Form 2, Somerville, Massachusetts, USA) with black resin (BLACK FLGPBL04, Formlabs, USA). A macro lens with tenfold magnification (APL-DG6 6in1, Apexel, China) was used as a part of the holder to ensure the highest image quality. The excitation was possible by using two LEDs λ = 400–410 nm (OSV5DL5111A, OptoSupply, Hong Kong, China) attached perpendicularly to the sides of the holder. LEDs (LED current was 20 mA) were powered by microcontroller (Metro Mini 328 5 V/16 MHz, Adafruit 2590, Adafruit, USA). The holder was attached to the smartphone so that the camera was perpendicular to the cuvette. A smartphone Samsung Galaxy S7, camera 12 Mpx (Suwon, South Korea) was used for the measurements.
Solutions for the measurements were prepared directly in 1 × 1 cm polymethyl methacrylate disposable cuvettes (Brand, Wertheim, Germany).
Analytical procedure
The calibration solutions of gentamicin were prepared in the range of 1.0–150 mg dm−3. These solutions were prepared in volumetric flasks by measuring an appropriate volume of gentamicin solution 10 mg cm−3 (Sigma-Aldrich, St. Louis, USA), and then the solutions were made up to 25 cm3 with deionised water. All solutions for measurements were prepared directly in 1 × 1 cm disposable cuvettes. Firstly, 0.25 cm3 of each of the prepared gentamicin standard solutions was transferred to the cuvettes (to prepare solutions diluted 10 times), and after the optimisation process, 0.05 cm3 of borate buffer (pH 8.5 or 9.5), and 0.20 cm3 of fluorescamine (1.5 g dm−3) were also introduced (to obtain the fluorescamine concentration in a cuvette of 0.12 g dm−3). To obtain a total volume of 2.5 cm3 of the solution for measurements, 2.0 cm3 of deionised water was added to cuvettes.
Drug samples were carefully homogenised with a test tube shaker, then properly diluted before addition to the cuvettes, and again thoroughly mixed before the analysis. After the optimised reaction time (3 min), measurements were performed spectrofluorimetrically (λex = 415 nm, λem = 489 nm) and using smartphone-based detection system (λex = 400–410 nm).
The accuracy of the method was determined as the relative error: |RE|, % = ((found value − expected value)/expected value × 100). In the case of analysis of drug samples RE—was calculated concerning the value declared by a manufacturer. Recovery values were calculated according to the equation: R, % = ((concentration determined in the spiked sample − concentration determined in the sample)/concentration added to the sample) × 100).
Procedure for smartphone-based detection
To perform measurements using smartphone-based detection, the Open Camera® software installed on a smartphone was used. It allowed control of the parameters of the camera settings (ISO, white balance, camera shutter) and was used to capture pictures of prepared solutions in cuvettes placed in the 3D printed holder printed with black resin. The project of this holder is shown in Fig. 5 and a picture of the entire measurement system is also available in Online Resource 2 (Supplementary Material).
The cuvette with the solution was placed in a dedicated part of the holder, to which two LEDs of the appropriate wavelength range were attached, positioned opposite each other to increase the intensity of the excitation radiation. To maintain the same conditions of measurements a black cap was put on the cuvette to reduce light exposure completely. In addition, pictures were captured in a dark room with reduced daylight and artificial light.
The captured images were analysed using ImageJ® software installed on a computer. The analysis of the images resulted in values for three channels R, G, and B as colour representations. In the process of optimising the measurement conditions, an optimal channel was selected whose values represented the analytical signal. The optimisation process also enabled the selection of appropriate parameters for the smartphone camera (ISO parameter, white balance, camera shutter). Five digital images were recorded for each solution in each experiment, and their signals were averaged.
Data availability
Data will be made available on request.
References
Tiwari A, Sharma P, Vishwamitra B, Singh G (2021) Coatings 11:1006
Weinstein MJ, Luedemann GM, Oden EM, Wagman GH, Rosselet JP, Marquez JA, Coniglio CT, Charney W, Herzog HL, Black J (1963) J Med Chem 6:463
Chen C, Chen Y, Wu P, Chen B (2014) J Formos Med Assoc 113:72
Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker JN (2018) Paediatr Int Child Health 38:S1
Walker SE, Coates PE (1981) J Chromatogr 223:131
Vučićević-Prčetić K, Cservenák R, Radulović N (2011) J Pharm Biomed Anal 56:736
Kowalczuk D, Pietraś R, Paw B, Czerkies A (2010) Pol J Environ Stud 19:587
Stypulkowska K, Blazewicz A, Fijalek Z, Sarna K (2010) Chromatographia 72:1225
Jariwala FB, Hibbs JA, Zhuk I, Sukhishvili SA, Attygalle AB (2020) J Anal Sci Technol 11:2
Donegatti TA, Lobato A, Duek EAR, Moreira Gonçalves L, Alves Pereira E (2020) Electrophoresis 41:1576
Jin Y, Jang JW, Han CH, Lee MH (2005) J Agric Food Chem 53:7639
Ghinami C, Giuliani V, Menarini A, Abballe F, Travaini S, Ladisa T (2007) J Chromatogr A 1139:53
Masihpour N, Hassaninejad-Darzi SK (2023) J Inorg Organomet Polym 33:1317
Krzek J, Woltyńska H, Hubicka U (2009) Anal Lett 42:473
Fraihat S (2015) Int J Pharm Sci 7:302
Habbal ZM (1979) Clin Chim Acta 95:301
Omar MA, Nagy DM, Hammad MA, Aly AA (2013) AAPS PharmSciTech 14:828
Fernández-Ramos JM, García-Campaña AM, Alés-Barrero F, Bosque-Sendra JM (2006) Talanta 69:763
McCracken KE, Tat T, Paz V, Yoon J-Y (2017) RSC Adv 7:9237
Hong JI, Chang B-Y (2014) Lab Chip 14:1725
Balbach S, Jiang N, Moreddu R, Dong X, Kurz W, Wang C, Dong J, Yin Y, Butt H, Brischwein M, Hayden O, Jakobi M, Tasoglu S, Koch AW, Yetisen AK (2021) Anal Methods 13:4361
Mermer K, Paluch J, Kozak J (2022) Monatsh Chem 153:801
Yang Y, Xu F, Chen J, Tao C, Li Y, Chen Q, Tang S, Lee HK, Shen W (2023) Biosens Bioelectron 229:115233
Rao H, Liu W, He K, Zhao S, Lu Z, Zhang S, Sun M, Zou P, Wang X, Zhao Q, Wang Y, Liu T (2020) ACS Sustain Chem Eng 8:8857
Alshatteri AH, Omer KM (2022) Anal Methods 14:1730
Komatsu T, Maeki M, Ishida A, Tani H, Tokeshi M (2020) ACS Sens 5:1287
Karlsen H, Dong T (2017) IEEE Trans Biomed Circuits Syst 11:455
Lu Y, Shi Z, Liu Q (2019) Curr Opin Food Sci 28:74
Sivakumar R, Lee NY (2021) Chemosphere 275:130096
Özdemir GK, Bayram A, Kılıç V, Horzum N, Solmaz ME (2017) Anal Methods 9:579
Moehling TJ, Lee DH, Henderson ME, McDonald MK, Tsang PH, Kaakeh S, Kim ES, Wereley ST, Kinzer-Ursem TL, Clayton KN, Linnes JC (2020) Biosens Bioelectron 167:112497
Lin B, Yu Y, Cao Y, Guo M, Zhu D, Dai J, Zheng M (2018) Biosens Bioelectron 100:482
Yu Z, Liao Y, Liu J, Wu Q, Cheng Y, Huang K (2023) Anal Methods 15:4282
Chen Y, Yu Q, Xu C-B (2017) Int J Clin Exp Med 10:14904
Hartig SM (2013) Curr Protoc Mol Biol 102:14.15.1
Tekkeli SE, Önal A, Sağırlı AO (2014) Luminescence 29:87
Granica M, Tymecki Ł (2019) Talanta 197:319
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubacki, K., Mermer, K., Paluch, J. et al. Spectrofluorimetric and smartphone-based detection methods for determination of gentamicin. Monatsh Chem 155, 899–909 (2024). https://doi.org/10.1007/s00706-024-03244-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-024-03244-x