Abstract
The reaction involving benzyl alcohol or benzyl halide and urea, conducted within a mixture comprising choline chloride and aluminium nitrate ionic liquid as the solvent, leads to the synthesis of N-monosubstituted urea products. The reaction demonstrates notable to exceptional yields for various derivatives of benzyl alcohols and benzyl halides under the specified reaction conditions. When benzyl halides and benzyl alcohol undergo reactions with urea in the presence of the prepared ionic liquid, a sole selective pathway is observed, resulting in a considerable yield of N-monosubstituted urea products. This approach offers several advantages, including reaction selectivity, high product efficiency, facile separability of products, the environmentally benign nature of the solvent, and the omission of expensive and hazardous catalysts. These attributes underscore the significance of such pioneering reactions. Due to the markedly polar attributes and insolubility of the resulting products in water, facile separation from the reaction milieu is facilitated through the introduction of water into the reaction mixture. Consequently, this specific ionic liquid methodology provides an uncomplicated and selective avenue for the synthesis of compounds possessing noteworthy pharmaceutical and industrial utility.
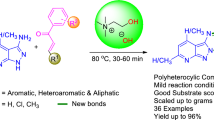
Graphical abstract

Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Abai M, Holbrey JD, Rogers RD, Srinivasan G (2010) New J Chem 34:1981
Artuso E, Degani I, Fochi R, Magistris C (2007) Synthesis 2007:3497
Azimian F, Hamzeh-Mivehroud M, Mojarrad JS, Hemmati S, Dastmalchi S (2020) Eur J Med Chem 201:112461
Bagherzadeh N, Sardarian AR, Inaloo ID (2021) New J Chem 45:11852
Bankston D, Dumas J, Natero R, Riedl B, Monahan M-K, Sibley R (2002) Org Process Res Dev 6:777
Basu P, Dey TK, Ghosh A, Biswas S, Khan A, Islam SM (2020) New J Chem 44:2630
Bigi F, Maggi R, Sartori G (2000) Green Chem 2:140
Burgess K, Ibarzo J, Linthicum DS, Russell DH, Shin H, Shitangkoon A, Totani R, Zhang AJ (1997) J Am Chem Soc 119:1556
Clark RL, Pessolano AA (1958) J Am Chem Soc 80:1657
De Luca L, Porcheddu A, Giacomelli G, Murgia I (2010) Synlett 2010:2439
Dombek BD, Angelici RJ (1977) J Organomet Chem 134:203
Doyle AG, Jacobsen EN (2007) Chem Rev 107:5713
Dragovich PS, Barker JE, French J, Imbacuan M, Kalish VJ, Kissinger CR, Knighton DR, Lewis CT, Moomaw EW, Parge HE (1996) J Med Chem 39:1872
Duvall JR, Wu F, Snider BB (2006) J Org Chem 71:8579
Enquist P-A, Nilsson P, Edin J, Larhed M (2005) Tetrahedron Lett 46:3335
Franz RA, Applegath F, Morriss F, Baiocchi F, Bolze C (1961) J Org Chem 26:3309
Funabashi Y, Tsubotani S, Koyama K, Katayama N, Harada S (1993) Tetrahedron 49:13
Gallou I (2007) Org Prep Proced Int 39:355
Garre S, Parker E, Ni B, Headley AD (2008) Org Biomol Chem 6:3041
Getman DP, DeCrescenzo GA, Heintz RM, Reed KL, Talley JJ, Bryant ML, Clare M, Houseman KA, Marr JJ (1993) J Med Chem 36:288
Giannoccaro P, Nobile CF, Mastrorilli P, Ravasio N (1991) J Organomet Chem 419:251
Gilbert A, Bucher G, Haines RS, Harper JB (2019) Org Biomol Chem 17:9336
González-Fernández R, Álvarez D, Crochet P, Cadierno V, Menéndez MI, López R (2020) Catal Sci Technol 10:4084
Gupte SP, Chaudhari RV (1988) J Catal 114:246
Habibi D, Heydari S, Faraji A, Keypour H, Mahmoudabadi M (2018) Polyhedron 151:520
Han F, Yang L, Li Z, Xia C (2012) Org Biomol Chem 10:346
Hansen BB, Spittle S, Chen B, Poe D, Zhang Y, Klein JM, Horton A, Adhikari L, Zelovich T, Doherty BW (2020) Chem Rev 121:1232
Haufe G, Rolle U, Kleinpeter E, Kivikoski J, Rissanen K (1993) J Org Chem 58:7084
Hawker RR, Haines RS, Harper JB (2018) Org Biomol Chem 16:3453
Hawker RR, Wong MJ, Haines RS, Harper JB (2017) Org Biomol Chem 15:6433
In SGMR (2005) Science of Synthesis, vol 18. Thieme Verlag, Stuttgart
Inaloo ID, Majnooni S (2018) New J Chem 42:13249
Jadhav VD, Herdtweck E, Schmidtchen FP (2008) Chem Eur J 14:6098
Jafari M, Heydari A (2022) J Mol Struct 1264:133267
Jefferson EA, Swayze EE (1999) Tetrahedron Lett 40:7757
Keaveney ST, Haines RS, Harper JB (2015) Org Biomol Chem 13:3771
Keaveney ST, White BP, Haines RS, Harper JB (2016) Org Biomol Chem 14:2572
Kim SH, Hong SH (2016) Org Lett 18:212
Knapp S, Hale JJ, Bastos M, Molina A, Chen KY (1992) J Org Chem 57:6239
Kumar K, Parveen F, Patra T, Upadhyayula S (2018) New J Chem 42:228
Lakkaniga NR, Zhang L, Belachew B, Gunaganti N, Frett B, Li H-y (2020) Eur J Med Chem 203:112589
Li F, Sun C, Shan H, Zou X, Xie J (2013) ChemCatChem 5:1543
Martins MA, Frizzo CP, Moreira DN, Zanatta N, Bonacorso HG (2008) Chem Rev 108:2015
Mathew T, Gurung L, Roshandel S, Munoz SB, Prakash GS (2019) Synlett 30:1037
Matsuda K (1994) Med Res Rev 14:271
McCusker JE, Main AD, Johnson KS, Grasso CA, McElwee-White L (2000) J Org Chem 65:5216
McHale KSS, Wong MJ, Evans AK, Gilbert A, Haines RS, Harper JB (2019) Org Biomol Chem 17:9243
Melnikov NN, Gaunther FA (2012) Chemistry of Pesticides. Springer- Verlag, New York
Mizuno T, Mihara M, Iwai T, Ito T, Ishino Y (2006) Synthesis 2006:2825
Nag S, Yadav G, Maulik P, Batra S (2007) Synthesis 911:917
Nasrollahzadeh M (2014) RSC Adv 4:29089
Nasrollahzadeh M, Azarian A, Ehsani A, Sajadi SM, Babaei F (2014) Mater Res Bull 55:168
Nasrollahzadeh M, Babaei F, Sajadi SM, Ehsani A (2014) Spectrochim Acta A Mol Biomol Spectrosc 132:423
Nasrollahzadeh M, Enayati M, Khalaj M (2014) RSC Adv 4:26264
Nasrollahzadeh M, Issaabadi Z, Sajadi SM (2018) RSC Adv 8:27631
Nasrollahzadeh M, Maham M, Sajadi SM (2015) J Colloid Interface Sci 455:245
Oh HK, Jin YC, Sung DD, Lee I (2005) Org Biomol Chem 3:1240
Orito K, Miyazawa M, Nakamura T, Horibata A, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Yamazaki T, Tokuda M (2006) J Org Chem 71:5951
Papesch V, Schroeder EF (1951) J Org Chem 16:1879
Peng X, Li F, Xia C (2006) Synlett 2006:1161
Rakesh K, Ramesha A, Shantharam C, Mantelingu K, Mallesha N (2016) RSC Adv 6:108315
Ran X, Long Y, Yang S, Peng C, Zhang Y, Qian S, Jiang Z, Zhang X, Yang L, Wang Z (2020) Org Chem Front 7:472
Regan J, Breitfelder S, Cirillo P, Gilmore T, Graham AG, Hickey E, Klaus B, Madwed J, Moriak M, Moss N (2002) J Med Chem 45:2994
Rogers RD, Seddon KR (2005) Ionic liquids IIIB: fundamentals, progress, challenges, and opportunities: transformations and processes. ACS Symposium Series, vol 902. ACS Publications, Washington, DC
Rosa NS, Glachet T, Ibert Q, Lohier J-F, Franck X, Reboul V (2020) Synthesis 52:2099
Schade D, Töpker-Lehmann K, Kotthaus J, Clement B (2008) J Org Chem 73:1025
Smith NW, Gourisankar SP, Montchamp J-L, Dzyuba SV (2011) New J Chem 35:909
Sroor FM, Othman AM, Tantawy MA, Mahrous KF, El-Naggar ME (2021) Bioorg Chem 112:104953
Tafesh AM, Weiguny J (1996) Chem Rev 96:2035
Tsopmo A, Ngnokam D, Ngamga D, Ayafor JF, Sterner O (1999) J Nat Prod 62:1435
Vishnyakova TP, Golubeva IA, Glebova EV (1985) Russ Chem Rev (Engl Transl) 54:249
Vyshnyakova T, Golubeva I, Glebova E (1985) Russ Chem Rev (Engl Transl) 54:249
Wang L, Wang H, Li G, Min S, Xiang F, Liu S, Zheng W (2018) Adv Synth Catal 360:4585
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis. John Wiley & Sons, Weinheim
Xu D, Ciszewski L, Li T, Repič O, Blacklock TJ (1998) Tetrahedron Lett 39:1107
Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN (2010) Science 327:986
Yoshida T, Kambe N, Murai S, Sonoda N (1986) Tetrahedron Lett 27:3037
Yu H-Z, Bencherif S, Pham-Truong T-N, Ghilane J (2022) New J Chem 46:454
Zhang Z, Schreiner PR (2009) Chem Soc Rev 38:1187
Zhu B, Angelici RJ (2006) J Am Chem Soc 128:14460
Zicmanis A, Katkevica S, Mekss P (2009) Catal Commun 10:614
Acknowledgements
We are grateful to Tarbiat Modares University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jafari, M., Darvishi, A. & Heydari, A. Synthesis of N-monosubstituted ureas in a mixture of choline chloride and aluminium nitrate as a simple, efficient, and selective process. Monatsh Chem 154, 1295–1306 (2023). https://doi.org/10.1007/s00706-023-03131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03131-x