Abstract
An ecologically safe and economically justified method of stabilization of the used biosorbents was developed. Sorbent contaminated with heavy metals has been successfully solidified/stabilized using a hydraulic binder. The test results indicated that up to 1% of the biosorbent residue used could be added without compromising the compressive strength of the mortar. The compressive strength of the modified mortars did not change significantly even after 20 freeze/thaw cycles. The analytical methods such as Flame Atomization-Atomic Absorption Spectrometer, Graphite Furnace-Atomic Absorption Spectrometer, and Cold Vapor-Atomic Absorption Spectrometry were utilized to examine the leaching behavior of selected heavy metals during harsh condition exposure. The leachability of selected heavy metals was found to be below the limit allowed by the US EPA after immersion and agitation for 10 days in artificial water solutions (seawater, groundwater, and rainwater). X-Ray Diffraction and Brunauer–Emmett–Teller data showed no significant changes in the crystalline structure and surface area of the modified mortars after treatment. Research showed that mixing the adsorbent used with mortar was effective in immobilizing heavy metals and allowed the implementation of a so-called ‘zero waste’ management method.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well-known that environmental pollution is commonly caused by natural sources and human activities. Broadly understood, human activities generate organic and inorganic wastes that can contain heavy metals that do not degrade naturally and cannot be utilized within simple waste treatment processes. Therefore, various researchers have developed treatment methods for heavy metals containing waste management to avoid environmental issues.
Adsorption is one of the basic and widely studied techniques used for wastewater treatment. Its popularity results from its simplicity, low implementation cost, and promptness of the process. The sorbents tested include agricultural by-products such as Garcinia mangostana L. [1], Arenga pinnata Merr. [2], Dimocarpus longan [3], and activated carbon from Quercus robur leaves [4], activated carbon from Phoenix dactylifera L. leaves [5], Sauropus androgynus (L.) Merr. [6], Camellia sinensis leaves [7], Citrus × paradisi peelings [8], Abelmoschus esculentus leaves [9], etc., as well as geomaterials such as clay, zeolite, and perlite [5, 7, 9,10,11].
An adsorption process involves interaction between adsorbates, functional groups, and other active sites (pores) that exist in adsorbents, such as hydroxyl, carbonyl, amine, and metal oxides. Other reactions that might occur in resin adsorbents are complex formation and ion exchange. The trapping in the pores also occurs in porous adsorbents such as zeolite and activated carbon [12]. Unfortunately, adsorption-based treatment processes result in solid and liquid post-process waste that needs to be properly managed. To regenerate the spent adsorbent after the adsorption process, alkaline, acid, or other desorbing agents are used. These release the adsorbates from the adsorbent making it reusable (desorption process). However, desorption produces liquids that contain adsorbates (hazardous materials), which makes the ‘zero waste’ approach impossible to implement in case of reuse of adsorbents. Implementing a proper method, to remove adsorbates from the desorbing agent, is necessary to avoid environmental damage; however, it is a difficult, complex, and economically unjustified process. This is considered to be the shortfall of the adsorption method. As the number of active sites inside the adsorbent is limited, if further desorption is impractical, a method to deal with spent adsorbents is needed.
Solid post-process waste could be used as an addition in mortar manufacture. Solidification/stabilization (S/S) processes in cement-based materials immobilize hazardous wastes and reduce the toxicity of these materials by improving stability and reducing solubility. The cement-based material acts as a hydraulic binder that traps contaminants within its matrix. Solidification/stabilization (S/S) is a simple process that allows toxic materials to be easily transported and stored, allowing the production of environmentally safe construction materials [13,14,15,16,17,18]. The approach based on the reuse of wastes in cement-based materials leads to avoiding unsterilized toxins that leach out of landfills (through the implementation of the eco-cement approach) [19, 20].
This approach allows the implementation of a so-called “zero waste” management method [21, 22]. The mentioned additions could be expected to improve the compressive strength of the mortar by filling the pores of the mortar [23, 24]. Potentially hazardous wastes such as radioactive waste [25], zinc (Zn(II)) [13], copper indium selenide (CIS), solar module wastes [26], arsenical borogypsum wastes [27], medical wastes [28], solid residues formed during sewage sludge thermal treatment [17], ceramic wastes [29], sugarcane bagasse [30], rice husk, disposed oil-contaminated soil [31], chromium-contaminated titania NPs [32], and coconut fibres [33] have all been trialled as incorporations into either cement matrices or concretes. The modified materials obtained have shown improved compressive strength and low leachability of metal ions. Aliyu and Karim [34] discovered that the compressive strength of a mortar material reached the optimal value when 15–20% of rice husk ash was added to the mixture. Rice husk ash is rich in SiO2, which is required in C–S–H formation, so the addition of rice husk ash supplemented the SiO2 available from the cement itself. Whereas Husain et al. [32] modified mortar with 20% wt. of adsorbed chromium in addition to titania nanoparticles found that the compressive strength of the modified mortar was improved. Stabilization/solidification in a cement matrix also reduces the mobility and leachability of hazardous elements, especially metal ions. Cement matrices have poor permeability and could hinder the movement of contaminants through pores [20]. Furthermore, biosorbents affect the structure of mortar or concrete. The mentioned problem should always be considered when incorporating wastes into a cement-based material matrix.
In addition to adsorbent addition, geographical conditions, such as extreme temperature fluctuations, contribute to mortar deterioration. Temperature changes, erosion, alkalinity, or infiltration of rain or seawater into mortar/concrete pores could result in the leakage of toxic material into the soil or water bodies. The degree to which this occurs depends on cement hydration and, in the case of heavy metals, the ability of ions to form complexes and precipitates. Elements such as As, Sb, and Cd can be bound in a silicate mineral crystal lattice to form a stable compound that prevents leaching. The leaching of detectable levels of Cu, Pb, Zn, Mn, and Sb can be prevented when these heavy metals have been encapsulated and solidified as a consequence of a cement hydration reaction [35]. According to Shi and Kan [19], the problem of leaching is greater in the case of materials stabilized for a short period of time due to the high concentration of potentially leachable heavy metals and low mechanical solidification on the surface of the matrix. The leachable concentration decreases with age as the mortar hardens [19]. Vollpracht and Brameshuber [36] found that K2CrO4 and K2Cr2O7 formed during clinker combustion could be immobilized in an ettringite structure, while Pb was able to react with Ca2+ to form an ettringite mineral [36].
Extreme weather conditions could also cause crystalline reduction and microcracks formation in the mortar. Micropores can transform into mesopores and macropores that affect mechanical properties [37]. Therefore, performing the crack test after exposure of a mortar to extreme conditions is important to estimate the effect of sorbents on the end products [38, 39]. At low temperatures, the water molecules expand up to 9%. This phenomenon can lead to the brittleness of the mortar. Since the adsorbent contained various heavy metals, the leaching of the mentioned ions from the mortar should also be controlled with the use of proper analytical techniques [28, 35, 36, 40].
Therefore, this research aimed to modify the mortar composition with sago bark adsorbent residue that contained heavy metals and investigated the leachability of the mentioned ions and the effect of extreme conditions (numerous freeze/thaw cycles) on its compressive strength together with checking if variety of atomic absorption spectrometry techniques would be suitable to solve given problem. The presented study aimed to evaluate the hazards associated with the addition of biosorbents containing heavy metals to cement-based materials with the use of Flame-Atomic Absorption Spectrometry (F-AAS), Graphite Furnace-Atomic Absorption Spectrometry (GF-AAS), Cold Vapour-Atomic Absorption Spectrometry (CV-AAS). Moreover, the amount of mentioned biosorbents that can be added to cement-based materials without significant deterioration of the end product to establish an ecologically safe and economically justified approach for stabilization was estimated. Physicochemical characterization of the mortars obtained including X-ray diffraction (XRD), analysis X-ray fluorescence (XRF) assay, and specific surface area (BET) analysis was investigated for further support of the presented study.
Results and discussion
For a better understanding of the properties of the mortars obtained, several tests and analyzes were performed, including the compressive strength test, the heavy metal leachability test and the freeze / thaw cycle (FTC) analysis. Materials and methods with the suitability of the analytical tools used are presented in the Experimental chapter. The findings of this research are explained in the following sections.
Effect of mortar composition on compressive strength
The utilization of biosorbent residue as an additive material in mortar production was meant to find a way to immobilize or solidify waste after metal ion sorption without compromising the durability of construction materials obtained. The effect of the biosorbent additive on the compressive strength of the mortar after 28 days of curing, without any additional treatment, is shown in Fig. 1. The amount of cement, sand and water was kept constant, but the biosorbent residue varied with the weight of the cement.
The compressive strength of the mortars was 23.9 ± 3.1 MPa for reference, 22.8 ± 3.2 MPa for 0.5% addition, 24.92 ± 0.73 MPa for 1% addition, 18.9 ± 1.1 MPa and 19.1 ± 1.4 MPa for 3% and 5% addition, respectively. The compressive strength of the modified mortar decreased as the percentage of biosorbent increased, due to its noticeable composition change. A greater proportion of biosorbents interfered with the hydration reaction [32]. Up to a certain percentage, the adsorbent did not have a negative impact on mortar durability. According to [41], it is possible to add even 10% of the sewage sludge ash without a relevant deterioration of the final cement-based material. Biosorbents, which are considered to be an undoubtly different additive, can be stabilized in cement-based materials in significantly lower amounts without observable harmful effects.
As mentioned above, the amount of waste that was utilized within the stabilized materials determines the mechanical strength, such as the compressive strength of the material. The cement consisted of tricalcium silicate (C3S), dicalcium silicate (C2S), tricalcium aluminate (C3A), and tetracalcium aluminate (C4AF). Tricalcium silicate (C3S) and dicalcium silicate (C2S) contributed to the compressive strength of the mortar. The cement hydration reaction occurred when SiO2 and CaO reacted with water forming calcium silicate hydrate (C–S–H). Calcium silicate hydrate plays a significant role in the durability of the mortar. The following reaction was described below [28, 42, 43]:
The compressive strength of the mortar is affected by the formation of C–S–H gels [28, 30]. The C–S–H gel forms due to the hydration of cement components such as C3S and C3A and is the main component that contributes to the compressive strength and influences the microstructure and properties of the mortar [44]. Therefore, the compressive strength decreases when the cement components are unable to react completely. In the case of the present study, the heavy metals contained in the biosorbents could have interacted with calcium hydroxide, forming insoluble metal hydroxides that hinder cement hydration [45]. Vysvaril et al. [45] indeed found an excessive amount of heavy metal in the mixture, which decreased the compressive strength of the samples. The reaction between heavy metals and Ca(OH)2 formed insoluble metal hydroxides preventing hydration. Furthermore, the increase in the material produced resulted in the cement not being able to bind all components of the mixture. Akyıldız et al. [28] in their study of solidification/stabilization (S/S) of medical waste incineration bottom ash found that the compressive strength decreased if the cement was doped with 50% of medical waste incineration bottom ash. The addition or replacement of other components to cement-based materials could either decrease or enhance the compressive strength. Therefore, it is necessary to consider the amount of cement and additives to establish the right composition in the formation [28, 34, 46,47,48].
Leaching test
Leaching tests were performed to observe the stability of metal ions in the mortar when exposed to different rough conditions. Metal leachability was carried out using mortar cubes that have 5 × 5 × 5 cm in size with various additions of biosorbent residues (0, 1, and 5%). This composition was chosen for further work as 1% and 5% represented the highest and the lowest compressive strength compared to the reference sample (0% biosorbent addition as the control).
The leachability of mortar containing metal ions was carried out by immersion of mortar in artificial groundwater, seawater, and rainwater [35, 49]. The concentration of leached metal ions, as well as their limit of detection (LOD) and limit of quantification (LOQ) values, are given in Table 1. The LOD and LOQ obtained were below the environmental standard value that the US Environmental Protection Agency has established. This implied that AAS could provide data on stabilization safety. Moreover, the atomic absorption spectrometer was considered as a simple and low-cost instrumentation for heavy metal determination compared to other instrumentations such as ICP-based techniques. The techniques that LODs were considered sufficient for environmental standards control are also presented in Table 1 as superscripts.
The leachability of mortar components was strongly affected by mortar pores [26]. The concentration of metal ions released into the liquid (groundwater, seawater, and rainwater) in almost every case was below LOD/LOQ after 10 days of immersion and agitation (Table 1). This result showed that hazardous heavy metals were successfully immobilized in mortar in all samples. Metals probably formed stable compounds due to cement hydration, ion exchange, complexation, sorption, or precipitation [35, 50]. The alkalinity of cementitious materials reduced the solubility of heavy metals, therefore, pollutants leached at negligibly low or minor concentrations [51, 52]. When the amount of residue content increased, the mortar cubes became brittle, as evidenced by a lower compressive strength and might slightly increased leachability [53]. However, the fact was not confirmed in the research. Immersion of mortar in various types of liquid initiated the reaction of ions that exist in the liquid to react with the mortar component, forming harmful compounds that later reduced the durability of the mortar [54, 55].
The concentration of Pb and Cr in the liquid phase was determined using both F-AAS and GF-AAS to ensure that their concentration was within the permissible limit. Among all tested heavy metals, only chromium was leached into the liquid phase after exposure to artificial seawater, groundwater, and rainwater in quantities exceeding the LOD/LOQ. However, the difference in Cr concentration was not statistically significant (P value > 0.05) for the various mortar compositions (Table 1). It indicated that the reference samples contained Cr. The Cr concentration in the reference sample was lower than in other samples due to the addition of bisorbent spent into the mortar. Thus, the leak did not necessarily originate from the biosorbent. It might come from cement processing or sand, which also proved a satisfactory degree of stabilization. Chromium could exist in both, + 3 and + 6 oxidation states due to clinker combustion [36]. The leached concentration of chromium was, although below the US Environmental Protection Agency's permissible limit of 5 mg dm−3 for total chromium in wastewater. It appears that the chromium ions were not able to be perfectly bound by the mortar components. Whereas, the metal ions inside the mortar require more time to leak out due to higher bonding with the material, longer diffusion paths, cement hydration, and higher pH in the mortar [35, 56, 57].
The C–S–H gel in the cement matrix was able to bind metal ions or form a precipitate because of the interaction between metal ions and either calcium or silicate. Therefore, the leachable concentration in the liquid phase decreased [14, 56, 58]. Dissociation of the dissolved hydrogen carbonate that forms the CO32− ion allowed calcium on the mortar surface or in the solution to produce CaCO3 precipitate. The CaCO3 precipitation hindered metal ion leaching [53]. Furthermore, the low concentration of metal ions that diffuse from mortar in this study indicates that metal ions have been encapsulated/solidified in mortar, forming stable compounds due to cement hydration that reduces the concentration of heavy metals in leachate to negligible levels [17, 35].
Although the addition of biosorbent residue reduced the compressive strength, the increase in the cement-to-waste ratio did not let metal ions leach, despite the change in the composition of the mortar. It is worth mentioning that some elements that were not introduced into the cement-based material matrix with biosorbents, however, which were present in the sample matrix, also did not leach. This only proves the stability of research materials. The data obtained give a good perspective to develop an ecologically safe method for the management of used biosorbents without noticeable deterioration of the durability of the final cement-based product. Barbir et al. [13] studied the leaching behavior of Zn in a modified cement-based material with sludge containing heavy metals and zeolite as a metal ion binder and found that enhancement of the binder content minimized the leachable concentration of metal ions in a liquid medium. This meant that the metal ions were successfully immobilized in cement-based materials. Similarly, Shi et al. [19] in a study of the leachability of heavy metals from modified concrete with fly ash from solid waste incineration, it was found that weak mechanical strength in the early stages resulted in the leaching of heavy metals. However, as hydration progressed, the solidity of cement-based materials improved, reducing water penetration and preventing the leaching of metal ions [19].
Freeze/thaw test
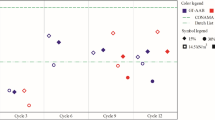
Freeze/thaw cycles (FTC) were performed to evaluate the resistance of the mortar after exposure to extreme conditions. Figure 2 presents the effect of freeze/thaw cycles on the durability of the modified mortar after 0, 10, and 20 cycles of FTC. The compressive strength of the reference samples was 26.8 ± 3.3 MPa at 0 cycles and 26.88 ± 0.41 MPa after 20 cycles. The compressive strength of a modified mortar with 1% addition was 26.4 ± 3.6 MPa at 0 cycles and 28.5 ± 4.3 MPa after 20 cycles. The modified mortar with 5% addition gained 21.3 ± 2.7 MPa of compressive strength at 0 cycles and 22.6 ± 1.7 MPa after 20 cycles.
It revealed that the freeze/thaw cycles provided a notable impact on the modified mortar with a 5% addition. This sample indicated a lower compressive strength compared to the reference sample (mortar without biosorbent addition). As mentioned previously, an appropriate composition was necessary to prevent brittleness [46]. Therefore, such harsh conditions (freeze/thaw cycles) led to a decrease in the compressive strength of the modified mortar with a 5% addition due to the lack of sufficient cement to maintain all components of the mortar. In addition, the freezing process allowed the water inside the mortar to freeze and create or widen the pores, yet the heating process allowed the water to fill the remaining unoccupied pores. Probably some microcracks could appear since the water increases its volume while freezing. Therefore, the compressive strength decreased for the sample with 5% biosorbent addition [38, 39, 59]. For all mortar samples tested with 0%, 1%, and 5% additive content, no significant differences in compressive strength were observed after exposure to FTC. It appeared that the biosorbent residue that was added to the mortar affected the compressive strength of the mortar. Freezing and thawing could result in microstructure changes and affect the leachability of heavy metals [59, 60]. Since the freeze/thaw process would be expected to leave unoccupied pores, the biosorbent residue may have occupied the pores. Keleştemur, et al. [61] stated that pores that were too small to be filled with sand might be able to be filled with smaller particle fillers while maintaining the mechanical strength of the mortar [61].
Furthermore, the mortar did not experience weight loss along with the freeze / thaw cycles (Fig. 3). This result indicated that the freeze/thaw cycles did not significantly affect the compressive strength of the mortar with different compositions even after 20 cycles (P value > 0.05). Matalkah et al. [62] found no decrease in compressive strength in concrete prepared with alkaline aluminosilicate cement after 30 and 60 freeze/thaw cycles. However, Wang et al. [60] study on the effect of freeze/thaw cycles on mixed cement containing co-combustion sewage sludge ash and rice husk has revealed that the compressive strength of each sample with a particular amount of modifier was reduced after 5 and 10 cycles due to water expansion during freeze/thaw cycles and the higher porosity of the modifier (co-combustion ash).
Mortar physicochemical characterization
The XRD diffractograms of the modified mortar after immersion and freeze/thaw cycles (0, 10, and 20 cycles) are illustrated in Fig. 4. The test was carried out with 3 repetitions and the error bar ( ±) represented the standard deviation. The patterns revealed that the mortar samples have consisted of various phases such as alite (Ca3SiO5), also known as C3S, albite (NaAlSi3O8), quartz (SiO2), dicalcium silicate (Ca2SiO4, known as C2S), mayenite (Ca12Al14O33), schwertmannite, and portlandite. The composition of the unmodified mortar (reference) after 20 FTC consisted of 52 ± 12% quartz, 42 ± 15% albite, and 6.2 ± 4.4% alite.
The modified mortar with 1% addition of biosorbent residue after 20 cycles of FTC contained 34 ± 10% quartz, 1.3 ± 1.0% alite, 26 ± 10% albite, 32 ± 8% schwertmannite, and 1.2 ± 0.6% portlandite. While, the composition of the modified mortar with a 5% addition (after 20 FTC) of biosorbent residue was 52 ± 20% quartz, 11.9 ± 8% alite, 30 ± 12% albite, 2.1 ± 1.4% mayenite, and 2.4 ± 0.8% schwertmannite. The freeze/thaw cycles appeared to have no impact on the crystal structure of the modified mortar and the unmodified mortar, since there were no significant changes between the XRD spectra after 0, 10, and 20 FTC. The treatment process did not trigger new peaks emerging, but contributed to the compressive strength (physical properties). Furthermore, the difference in concentration between reference and the modified mortar was not statically significant (P value > 0.05).
All assigned crystalline phases are commonly found in cement. The quartz content remained relatively high with the addition of residue and plays a key role in the formation of the CSH gel that reacts with cementitious materials that influence compressive strength [43, 44]. Schwertmannite and mayenite, which appeared in modified mortar samples, could be by-products of cement hydration, as well as the output from cement and residues [63,64,65,66]. Heavy metals were undetectable during XRD analysis because their concentrations were too low. According to Aliyu et al. [34], the heavy metals were able to establish metal hydrate or precipitation due to cement hydration, and the production of C–S–H assisted immobilization of heavy metals by trapping them in their structure [34, 67]. C3S and C2S were the main compounds that produced C–S–H and portlandite during cement hydration [42]. Therefore, it could be considered that heavy metals have been encapsulated within the cement structure.
The oxides compositions which are involved in the treatment process, were determined using XRF analysis. The results for selected mortars (unmodified (reference), modified (5% addition)) and spent biosorbent are shown in Table 2. The modification process changed the chemical composition of the mortar. It was hard to say whether the heavy metal came from cement, sand, or spent biosorbents. Therefore, a reference was set as a standard and the chemical composition of the biosorbent spent was presented as a comparison.
The previous studies [36, 67, 68] found that contaminants such as heavy metals were merged into the surface of C–S–H first before incorporation into its structure. Those heavy metals could form a precipitate due to the reaction with the cement component. Therefore, the selected heavy metals remained stable in the mortar structure and did not leak into the liquid medium [36, 67, 68].
The surface of the BET area and the pore volume of the unmodified and modified mortar (1% addition of biosorbent) are shown in Table 3. The addition of residues did not make a significant difference in these values. The surface area can affect the rate of hydration, which influences the physical and mechanical properties of the mortar. The BET surface area measurements indicated that 1% addition of the used adsorbent did not change the physical properties. This suggests that the selected heavy metals could be immobilized without further effect, indicating that the hydration process was completed [69].
Conclusions
The addition of 1% biosorbent residue from sago bark (Metroxylon sagu) did not significantly affect the quality of the cement-based materials obtained. The compressive strength remained at a satisfactory level even after immersion in artificial water (seawater, rainwater, and groundwater) and performing 20 freeze/thaw cycles. It is worth mentioning that the leachability test with the use of artificial rainwater, groundwater, and seawater revealed no, or an extremely low concentration of selected heavy metals that were leached. The concentration of leached heavy metals was below the LOD/LOQ in most cases, which can be considered satisfactory.
The high ratio of heavy metal stabilization has been preserved independently of the kind of water solution used for leaching, as they were well encapsulated in the cement structure, reducing the mobility of metal ions. The leachability of the selected heavy metals was below accepted environmental standards, indicating that up to 1% addition of sorbent to the mortar may be environmentally and economically feasible. Higher rates of biosorbent addition to mortar, reaching up to 3%, did negatively influence the strength and durability of the final product, however, the final product still met the durability requirements of American Society for Testing and Materials.
The research proved that the biosorbent containing metal ions could be effectively immobilized onto cement-based material (mortar) in specified amounts to overcome the environmental issue, with no significant loss in durability. It is worth mentioning that with such an approach, the issue of managing relatively small amounts of heavy metal-containing biosorbents can be effectively solved. However, the possibility of heavy metal leaching after significant periods of time should be evaluated. It is worth stating that waste-containing cement-based materials ‘at the end of life’ should be managed with special care, e.g., landfilled as hazardous waste. To help justify the economic point of view, it is suggested that the compressive strength and other durability parameters of mortars with biosorbent addition between 1 and 3% should be explored to determine if stabilizing larger amounts of biosorbent may be possible without significantly compromising the strength characteristics.
The presented study is a step towards developing a ‘zero waste’ approach to the management of used biosorbents and in terms of the preservation and aesthetic of the environment. Moreover, cheap and broadly available spectroscopic techniques were considered suitable and efficient enough to provide data on environmental hazards evaluation.
Experimental
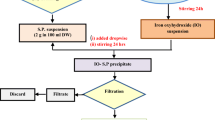
Portland Composite Cement (CEM II) produced by PT Semen Padang, Indonesia was used as the hydraulic binder. The used adsorbent was collected from previous work by [70,71,72]. The previous work employed sago bark as an adsorbent to remove heavy metals such as Cu(II), Pb(II), Cd(II), and Cr(VI) on a laboratory scale. The adsorption process was conducted using a batch method by submerging the adsorbent into a solution containing heavy metals. Then, the adsorbent used was dried at room temperature for further use [70, 71].
Mortar was immersed in artificial seawater, groundwater, and rainwater at a particular time, prepared according to [35, 73]. Each component was analytically prepared by diluting reagent in distillated water. Then the solutions were mixed and thoroughly stirred. The detailed compositions are indicated in Table 4. The composition of the rainwater was based on the molar ratio of H2SO4: HNO3 (3:1) which was diluted until pH equal to 5.6 [73].
Mortar preparation
The mortar composition was made using 250 g of cement, 750 g sand, and 151 cm3 of water. The water to cement ratio was equal to 0.604, according to the flow table analysis. 0, 0.5, 1, 3, and 5 wt. % of biosorbent containing metal ions were added into the prepared mixtures. These mixtures were mixed at 140 ± 5 rpm for 30 s using a mixer. It was paused for 15 s to remove the mixture from the mixer wall. The mixture was then mixed at 285 ± 10 rpm for 150 s. It was moulded into the 5 cm × 5 cm × 5 cm steel cube. The mixture cubes were then cured for 28 days at room temperature, according to the Standard Test Method no. ASTM C 109/109 M–02.
Compressive strength, leachability, and freeze/thaw cycle tests
The compressive strength, leachability and freeze/thaw cycle tests were conducted after 28 days of curing. The leachability test was carried out by immersion of mortar in artificial groundwater, seawater and rainwater with liquid to solid mass ratio (L/S) equal to 3 (approx. 750 cm3), at 113 ± 3 rpm of agitation speed for 10 days [74].
The concentration of metal ions such as silver, cadmium, cobalt, copper, iron, manganese, nickel, lead, strontium, zinc, chromium, arsenic, and mercury, which were released during agitation, was evaluated after 10 days [19, 35, 74]. Leached metal ion concentrations were determined using several types of equipment (wavelength and other parameters used for analysis of specific elements presented in curly brackets): Atomic Absorption Spectrophotometer with the Flame Atomization System (F-AAS) (acetylene fuel flow up to 2.0 dm3 min−1, air flow up to 8.0 dm3 min−1) SensAA DUAL, supplied by GBC Scientific Equipment (silver (328.10 nm, slit width 0.50 nm), cadmium (228.80 nm, slit width 0.50 nm), cobalt (240.70 nm, slit width 0.20 nm), copper (324.70 nm, slit width 0.50 nm), chromium (357.90 nm, slit width 0.20 nm), iron (248.30 nm, slit width 0.20 nm), manganese (279.50 nm, slit width 0.20 nm), nickel (232.00 nm, slit width 0.20 nm), lead (217.00 nm, slit width 1.00 nm), strontium (460.70 nm, slit width 0.20 nm), and zinc (213.90 nm, slit width 0.50 nm); Atomic Absorption Spectrophotometer with the Graphite Furnace Atomization System (GF-AAS) Savant AAZ (furnace ashing temp. for all elements of interest analysis 700–750 ℃, furnace read temp. for all elements of interest analysis 2500 ℃), supplied by GBC Scientific Equipment chromium (357.90 nm, slit width 0.20 nm), lead (217.00 nm, slit width 0.50 nm) and arsenic (193.70 nm, slit width 0.50 nm), Atomic Absorption Spectrometry coupled with Cold Vapour technique (CV-AAS) and Mercury Analyser MA-3000, supplied by Nippon Instruments Corporation max furnace temperature 850 ℃ for Hg (253,7 nm, slit width 0.20 nm) determination). All results are presented with the standard uncertainty (3–4 results). The concentration of nitric acid for all samples was fixed to prevent spectral overlap during measurement.
After immersion in artificial groundwater, seawater, and rainwater, the samples were exposed to extreme temperature fluctuations in the freeze/thaw cycle (FTC) analysis. The samples were frozen at − 35 °C for 24 h, then stabilized at room temperature for 10 min before being heated to 50 °C for the next 24 h. A full FTC was achieved within 48 h. The compressive strength test was carried out according to Standard Test Method no. ASTM C 109/109 M-02 and was investigated after 0, 10, and 20 FTC cycles. The mortar was placed on automatic compression tester equipment (Control-Pilot 4) giving pressure to the mortar until the mortar cracked [25]. The experimental data were statically analyzed using ANOVA single factor.
Physicochemical characterization
The crystal structure was investigated using XRD using a Rigaku Intelligent X-ray diffraction system SmartLab equipped with a sealed tube X-ray generator. Data were collected in the 2θ range 5–80°. The scan speed and the scan steps were 1 and 0.01° min−1, respectively. The analysis was based on ICDD database (reference card numbers for Albite, SiO2, Alite, Ca(OH)2, Schwertmannite and Mayenite are 9,000,526, 5,000,035, 9,016,125, 9,009,098, 9,015,185 and 9,011,737, respectively). Quantitative analysis including phase composition with standard deviation was calculated using the reference intensity ratio method from the most intense independent peak of each phase. Specific surface area measurement was determined using the Brunauer–Emmet–Teller (BET) method. The analysis was performed at liquid nitrogen temperature (77 K) using a Micromeritics Gemini V instrument (model 2365). The analysis was preceded by 2 h of degassing at 473 K (200 °C) using nitrogen gas to remove air from the pores of the sample. For each sample, BET surface area measurements were conducted 3 times, to evaluate the uncertainty. XRF analysis was used to determine the chemical composition of the mortar, using the Malvern PANalytical Epsilon 3 Spectrometer (30 kV, 300 μA, filter: Ag, medium: air).
References
Zein R, Suhaili R, Earnestly F, IndrawatyMunaf E (2010) J Hazard Mater 181:52–56. https://doi.org/10.1016/j.jhazmat.2010.04.076
Zein R, Hidayat DA, Elfia M, Nazarudin N, Munaf E (2014) J Water Supply Res Technol AQUA 63:553
Florenly IR, Aziz H, Syafrizayanti ZR (2015) J Chem Pharm Res 7:81
Sulyman M, Namiesnik J, Gierak A (2014) Polish J Environ Stud 23:2223
Sulyman M, Namiesnik J, Gierak A (2016) Eng Prot Environ 19:611
Ginting CN, Munaf E, Almahdy YE, Zei R (2015) J Chem Pharm Res 7:39
Cheraghi M, Sobhanardakani S, Zandipak R, Lorestani B, Merrikhpour H (2015) Iran J Toxicol 9:1247
Rosales E, Meijide J, Tavares T, Pazos M, Sanroman MA (2016) Process Saf Environ Prot 101:61
Khaskheli MI, Memon SQ, Chandio ZA, Jatoi WB, Mahari MT, Khokhar FM (2016) Am J Anal Chem 7:395
Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Lee YB, Naidu R (2016) Process Saf Environ Prot 100:173
Lee LY, Chin DZB, Lee XJ, Chemmangattuvalappil N, Gan S (2015) Process Saf Environ Prot 94:329
Gautam RK, Gautam PK, Banerjee S, Rawat V, Soni S, Sharma SK, Chattopadhyaya MC (2015) J Environ Chem Eng 3:79
Barbir D, Dabic P, Krolo P (2012) Hem Ind 66:781
Zhang H-Q, Yang Y-Y, Yi Y-C (2017) Trans Nonferrous Met Soc China 27:666
Cinquepalmi MA, Mangialardi T, Panei L, Paolini AE, Piga L (2008) J Hazard Mater 151:585
Mohd Zain A, Zazarin ZM, Azizli KA, Isa MH (2017) Appl Mech Mater 865:341
Cieślik BM, Zając M, Gałuszka A, Konieczka P (2018) J Clean Prod 178:757
Sahnoune R, Moussaceb K (2019) Nov Biotechnol Chim 18:166
Shi HS, Kan LL (2009) J Hazard Mater 164:750
Rubio-Cintas MD, Barnett SJ, Perez-García F, Parron-Rubio ME (2019) Constr Build Mater 204:675
Giergiczny Z, Krol A (2008) J Hazard Mater 160:247
Kotatkova J, Zatloukal J, Reiterman P, Kolar K (2017) J Environ Radioact 178–179:147
Al-Zubaidi AB (2015) Int J Sci Eng Res 6:76
Garcia L, Sousa-Coutinho J (2013) Constr Build Mater 41:897
Eskander SB, Aziz SMA, El-Didamony H, Sayed MI (2011) J Hazard Mater 190:969
Skripkiunas G, Vasarevicius S, Danila V (2018) Cem Concr Compos 85:174
Alp I, Deveci H, Sungun YH, Yazici EY, Savas M, Demirci S (2009) Environ Sci Technol 43:6939
Akyıldız A, Köse ET, Yıldız A (2017) Constr Build Mater 138:326
Reig L, Sanz MA, Borrachero MV, Monzó J, Soriano L, Payá J (2017) Ceram Int 43:13622
Rino A, Farida A, Elvaswer, Dahlan D. 2017 AIP Conf Proc. 180(1):p040005
Li Y, Zeng X, Zhou J (2020) Resour Conserv Recycl 160:104838
Husnain A, Qazi IA, Khaliq W, Arshad M (2016) J Environ Manage 172:10
Domke PV (2012) IOSR J Eng 2:755
Aliyu MK, Karim ATA (2016) ARPN J Eng Appl Sci 11:5365
Lu H, Wei F, Tang J, Giesy JP (2016) J Environ Manage 169:319
Vollpracht A, Brameshuber W (2016) Cem Concr Res 79:76
García-Giménez R, Frías M, Arribas I, Vegas I, de la Villa RV, Rubio V (2018) Constr Build Mater 190:140
Chowdhury S, Mishra M, Suganya O (2015) Ain Shams Eng J 6:429
Farahani JN, Shafigh P (2017) Mahmud H BIn. Procedia Eng 184:778–783
Shen D, Huang M, Feng H, Li N, Zhou Y, Long Y (2017) Waste Manag 61:345
Swierczek L, Cieslik BM, Konieczka P (2021) J Clean Prod 287:125054
Satiyawira B, Fathaddin MT, Setiawan R (2010) Proc World Geotherm Congr 2010
Limbachiya V, Ganjian E, Claisse P (2016) Constr Build Mater 113:273
Pina A, Ferrão P, Fournier J, Lacarrière B, Le CO (2017) Energy Procedia 139:689
Vyšvařil M, Bayer P (2016) Procedia Eng 151:162
Świerczek L, Cieślik BM, Konieczka P (2018) J Clean Prod 200:342
Gomes SDC, Zhou JL, Li W, Qu F (2020) Resour Conserv Recycl 161:104970
Thakare AA, Singh A, Gupta V, Siddique S (2021) Resour Conserv Recycl 168:105250
Yaseen SA, Yiseen GA, Poon CS, Li Z (2020) ACS Sustain Chem Eng 8:15875
Fang W, Wei Y, Liu J (2016) J Hazard Mater 310:1
Hu J, Wang K, Gaunt JA (2010) Resour Conserv Recycl 54:1453
Yoon I, Hyun D, Kim K, Lee K, Lee J, Gyu M (2010) J Environ Manage 91:2322
Hartwich P, Vollpracht A (2017) Cem Concr Res 100:423
Liu Z, Xu D, Gao S, Zhang Y, Jiang J (2020) ACS Sustain Chem Eng 8:3718
Ertelt MJ, Raith M, Eisinger J, Grosse CU, Lieleg O (2020) ACS Sustain Chem Eng 8:5704
Yu Q, Nagataki S, Lin J, Saeki T, Hisada M (2005) Cem Concr Res 35:1056
Gwenzi W, Mupatsi NM (2016) Waste Manag 49:114
Liu J, Zha F, Xu L, Kang B, Yang C, Zhang W, Zhang J (2020) Appl Clay Sci 186:105474
Uranjek M, Bokan-Bosiljkov V (2015) Constr Build Mater 84:416
Wang T, Xue Y, Zhou M, Lv Y, Chen Y, Wu S, Hou H (2017) Constr Build Mater 131:361
Keleştemur O, Yildiz S, Gökçer B, Arici E (2014) Mater Des 60:548
Matalkah F, Soroushian P (2018) Constr Build Mater 163:200
Gitari WM, Petrik LF, Key DL, Okujeni C (2011) J Environ Sci Heal Part A 46:117
Zhang Z, Bi X, Li X, Zhao Q, Chen H (2018) RSC Adv 8:33583
Gabrovšek R, Vuk T, Kaučič V (2006) Acta Chim Slov 53:159
He Z, Li Y (2018) Materials (Basel) 11:1958
Guo B, Liu B, Yang J, Zhang S (2017) J Environ Manage 193:410
Verbinnen B, Block C, Van Caneghem J, Vandecasteele C (2015) Waste Manag 45:407
Mantellato S, Palacios M, Flatt RJ (2015) Cem Concr Res 67:286
Fauzia S, Aziz H, Dahlan D, Namiesnik J, Zein R (2019) Desalin Water Treat 147:191
Fauzia S, Aziz H, Dahlan D, Zein R (2018) AIP Conf Proc 2023:020081
Fauzia S, Aziz H, Dahlan D, Zein R (2019) Rasayan J Chem 12:1889
Zhao C, Ren S, Zuo Q, Wang S, Zhou Y, Liu W, Liang S (2018) Sci Total Environ 627:553
Dell M, Mangialardi T, Evangelista A, Piga L (2012) J Hazard Mater 227:1–8
Acknowledgements
This work was supported by the Directorate General of Higher Education, Ministry of Research Technology and Higher Education of Republic Indonesia; No. Grant: 050/SP2H/LT/DPRM/2018. The Authors would like to give special thanks to Prof. Jacek Namieśnik as a former rector and head of the Department of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, Poland, who passed away in April 2019, for support during research and manuscript preparation.
Funding
Direktorat Riset Dan Pengabdian Kepada Masyarakat, 050/SP2H/LT/DPRM/2018, Rahmiana Zein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zein, R., Fauzia, S., Bielan, Z. et al. Analytical chemistry in technical approaches: immobilization of biosorbent waste containing heavy metals in cemented materials. Monatsh Chem 153, 789–800 (2022). https://doi.org/10.1007/s00706-022-02963-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02963-3