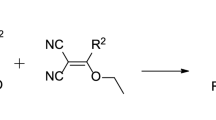
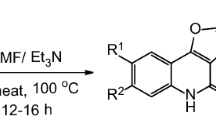
Abstract
Synthesis of heteroannulated pyrano[3,2-c]quinolones was established starting from the reaction of 4-hydroxyquinolin-2-ones with ethene-1,2,3,4-tetracarbonitrile. Several conditions were carried out, and the corresponding product yields were illustrated. The neutral and non-polar condition was the best procedure for product formation. The structure of products was elucidated by NMR, IR, mass spectra, and elemental analysis. X-ray structure analysis was also used to elucidate the structure of the obtained products. The mechanism of products formation was also discussed.
Graphical abstract




Similar content being viewed by others
References
Elshaier Y, Aly AA, Abd El-Aziz MA, Fathy H, Brown AB, Ramadan M (2022). Mol Div. https://doi.org/10.1007/s11030-021-10332-1
Wabo HK, Tane P, Connolly JD, Okunji CC, Schuster BM, Iwu MM (2005) Nat Prod Res 19:591
Danaei G, Vander Hoorn S, Lopez A, Murray C, Ezzati M (2005) Lancet 366:1784
Ramesh M, Mohan P, Shanmugam P (1984) Tetrahedron 40:4041
Ea S, Giacometti S, Ciccolini J, Akhmedjanova V, Aubert C (2008) Planta Med 74:1265
Dhiman P, Arora N, Thanikachalam PV, Monga V (2019) Bioorg Chem 92:103291
Ramadan M, Elshaier YA, Aly AA, Abdel-Aziz M, Fathy HM, Brown AB, Pridgen JR, Dalby KN, Kaoud TS (2021) Bioorg Chem 105344
Kumar NP, Thatikonda S, Tokala R, Kumari SS, Lakshmi UJ, Godugu C, Shankaraiah N, Kamal A (2018) Bioorg Med Chem 26:1996
Wang X-S, Zhang M-M, Zeng Z-S, Shi D-Q, Tu S-J, Wei X-Y, Zong Z-M (2005) Chem Lett 34:1316
El-Taweel FM, Elagamey A-GA, Khalil MH (2013) Chem Sci Int J 532
Dodia N, Shah A (2001) Heterocycl Commun 7:289
Gunasekaran P, Prasanna P, Perumal S, Almansour AI (2013) Tetrahedron Lett 54:3248
Zhu S, Wang J, Xu Z, Li J (2012) Molecules 17:13856
El-Sheref EM, Aly AA, Mourad A-FE, Brown AB, Bräse S, Bakheet ME (2018) Chem Pap 72:181
Abass M, Mohamed EA, Ismail MM, Mayas AS (2011) J Mex Chem Soc 55:224
Aly AA, El-Sheref EM, Mourad A-FE, Brown AB, Bräse S, Bakheet ME, Nieger M (2018) Monatsh Chem 149:635
Majumdar K, Mukhopadhyay P (2003) Synthesis 97
Kupwade V, Kulkarni A, Lad U (2022). Polycycl Arom Compds. https://doi.org/10.1080/10406638.2021.2015398
Buckle DR, Cantello BC, Smith H, Spicer BA (1975) J Med Chem 18:726
Sheldrick G (2015) Acta Cryst A 71:3
Sheldrick G (2015) Acta Cryst C 71:3
Acknowledgements
The authors thank DFG for providing Ashraf A. Aly with a fellowship, enabling him to conduct the compound analysis at the Karlsruhe Institute of Technology, Karlsruhe, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated for the memory of Professor Dr. Raafat Mohamed Shaker.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aly, A.A., El-Naby, H.A.A., Ahmed, E.K. et al. Facile synthesis of new pyrano[3,2-c]quinolones via the reaction of quinolin-2-ones with ethene-1,2,3,4-tetracarbonitrile. Monatsh Chem 153, 277–284 (2022). https://doi.org/10.1007/s00706-022-02903-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02903-1