Abstract
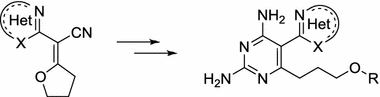
Synthetic approaches for the preparation of 6-amino-5-hetarylpyrimidine derivatives by the ring transformation reaction of 2-hetaryl-2-(tetrahydro-2-furanyliden)acetonitriles with amidines have been developed. Some of 2,6-diamino-5-(1,3-benzothiazol-2-yl)pyrimidines were found to exhibit modest inhibitory activity against human dihydrofolate reductase. Molecular docking was performed to evaluate the binding mode of compounds of this series in the enzyme’s active site.
Graphical abstract


Similar content being viewed by others
References
Kompis IM, Islam K, Then RL (2005) Chem Rev 105:593
Arooj M, Sakkiah S, Ping Cao G, Lee KW (2013) PLoS One 8:e60470
Jackson RC (1999) Antifolate drugs: past and future perspectives. In: Jackman AL (ed) Antifolate drugs in cancer therapy. Humana Press Inc, Totowa
Sharma M, Chauhan PMS (2012) Future Med Chem 4:1335
Bag S, Tawari NR, Degani MS, Queener SF (2010) Bioorg Med Chem 18:3187
Gangjee A, Li W, Lin L, Zeng Y, Ihnat M, Warnke LA, Green DW, Cody V, Pace J, Queener SF (2009) Bioorg Med Chem 17:7324
Gangjee A, Jain HD, Phan J, Guo X, Queener SF, Kisliuk RL (2010) Bioorg Med Chem 18:953
Zhang X, Zhou X, Kisliuk RL, Piraino J, Cody V, Gangjee A (2011) Bioorg Med Chem 19:3585
Gibson CL, Huggan JK, Kennedy A, Kiefer L, Lee JH, Suckling CJ, Clements C, Harvey AL, Hunter WH, Tulloch LB (2009) Org Biomol Chem 7:1829
Algul O, Paulsen JL, Anderson AC (2011) J Mol Graph Model 29:608
Chowdhury SF, Guerrero RH, Brun R, Ruiz-Perez LM, Pacanowska DG, Gilbert IH (2002) J Enz Inhib Med Chem 17:293
Nammalwar B, Bourne CR, Wakeham N, Bourne PC, Barrow EW, Muddala NP, Bunce RA, Berlin KD, Barrow WW (2015) Bioorg Med Chem 23:203
Muddala NP, Nammalwar B, Selvaraju S, Bourne CR, Henry M, Bunce RA, Berlin KD, Barrow EW, Barrow WW (2015) Molecules 20:7222
Russell PB, Hitchings GH (1951) J Am Chem Soc 73:3763
Hung J, Werbel LM (1984) J Het Chem 21:741
Haddow J, Suckling CJ, Wood HCS (1987) J Chem Soc Chem Commun 6:478
Kamchonwongpaisan S, Quarrell R, Charoensetakul N, Ponsinet R, Vilaivan T, Vanichtanankul J, Tarnchompoo B, Sirawaraporn W, Lowe G, Yuthavong Y (2004) J Med Chem 47:673
Stenbuck PA, Baltzly R, Hood HM (1963) J Org Chem 28:1983
Tarnchompoo B, Sirichaiwat C, Phupong W, Intaraudom C, Sirawaraporn W, Kamchonwongpaisan S, Vanichtanankul J, Thebtaranonth Y, Yuthavong Y (2002) J Med Chem 45:1244
Berber H, Mirand C, Derouin F (2007) J Fluor Chem 128:1039
Pez D, Leal I, Zuccotto F, Boussard C, Brun R, Croft SL, Yardley V, Perez LMR, Pacanowskab DG, Gilbert IH (2003) Bioorg Med Chem 11:4693
El-Hamamsy MHRI, Smith AW, Thompson AS, Threadgill MD (2007) Bioorg Med Chem 15:4552
Pattabiraman K, El-Khouri R, Modi K, McGee LR, Chow D (2009) Tetrahedron Lett 50:1571
Pätzel M, Liebscher J (1995) Synthesis:879
Neidlein R, Li S (1996) J Heterocycl Chem 33:1943
Neidlein R, Li S (1995) Synth Commun 25:2379
Sosnicki JG (2000) Monatsh Chem 131:475
Bellur E, Langer P (2006) Tetrahedron 62:5426
Kralj D, Groselj U, Meden A, Dahmann G, Stanovnik B, Svete J (2007) Tetrahedron 63:11213
Maximov NB, Mykhailiuk PK, Golovach SM, Tverdokhlebov AV, Tolmachev AA, Voitenko ZV (2010) Synthesis:1781
Maximov NB, Mykhailiuk PK, Kisel AI, Voitenko ZV, Tolmachev AA (2011) Synthesis 9:1465
Shvidenko KV, Nazarenko KG, Shvidenko TI, Tolmachev AA (2010) Chem Heterocycl Compd 46:56
Stepaniuk OO, Matvienko VO, Mykhailiuk PK, Tolmachev AA, Kondratov IS, Volochnyuk DM, Shishkin OV (2012) Synthesis 44:895
Bonacorso HG, Porte LMF, Paim GR, Luz FM, Martins MAP, Zanatta N (2010) Tetrahedron Lett 51:3759
Zanatta N, Amaral SS, Esteves-Souza A, Echevarria A, Brondani PB, Flores DC, Bonacorso HG, Flores AFC, Martins MAP (2006) Synthesis 14:2305
Hitchcock PB, Rahman S, Young DW (2003) Org Biomol Chem 1:2682
Hitchcock PB, Papadopoulos K, Young DW (2003) Org Biomol Chem 1:2670
Coe D, Drysdale M, Philps O, Westa R, Young DW (2002) J Chem Soc Perkin Trans 1:2459
Montero A, Feist H, Michalik M, Quincoces J, Peseke K (2002) Synthesis 5:664
Bari A, Milicevic S, Feist H, Michalik D, Michalik M, Peseke K (2005) Synthesis 16:2758
Khilya OV, Volovnenko TA, Turov AV, Zubatyuk RI, Shishkin OV, Volovenko YM (2013) Chem Heterocycl Compd 48:1770
Khilya OV, Milokhov DS, Postupalenko VY, Turov AV, Volovenko YM (2013) Monatsh Chem 144:1071
Milokhov DS, Khilya OV, Turov AV, Medviediev VV, Shishkin OV, Volovenko YM (2014) Tetrahedron 70:1214
Milokhov DS, Khilya OV, Volovenko YM, Palamarchuk GV, Shishkin OV (2012) Synlett 23:2063
Khilya OV, Volovnenko TA, Turov AV, Zubatyuk RI, Shishkin OV, Volovenko YM (2011) Chem Heterocycl Compd 47:1141
Cody V, Luft JR, Pangborn W (2005) Acta Cryst D 61:147
Lamb KM, G-Dayanandan N, Wright DL, Anderson AC (2013) Biochemistry 52:7318
Reynolds RC, Campbell SR, Fairchild RG, Kisliuk RL, Micca PL, Queener SF, Riordan JM, Sedwick WD, Waud WR, Leung AKW, Dixon RW, Suling WJ, Borhani DW (2007) J Med Chem 50:3283
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khilya, O.V., Milokhov, D.S., Kononets, L.A. et al. Synthesis and evaluation of new 2,6-diamino-5-hetarylpyrimidines as inhibitors of dihydrofolate reductase. Monatsh Chem 149, 813–822 (2018). https://doi.org/10.1007/s00706-017-2032-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2032-7