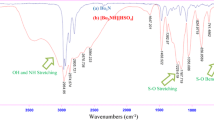
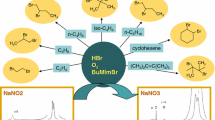
Abstract
Sodium bromate with bmim[HSO4] has been found to be an excellent oxidizing agent in aqueous medium. NaBrO3:bmim[HSO4] oxidized 1,2-diols, α-hydroxyketones, and alcohols to the corresponding carbonyl compounds in excellent yields. This method offers advantages such as low cost reagents, aqueous reaction conditions, moderate temperatures and short reaction times and hence environmentally benign reaction. Moreover, the ionic liquid bmim[HSO4] could be recycled for at least three times without loss of significant activity.
Graphical abstract


Similar content being viewed by others
References
Richardson WH (1965) In: Wiberg KB (ed) Oxidation in Organic Chemistry, Part A, Ch. IV. Academic Press, New York
Ojha LR, Kudugunti S, Maddukuri PP, Kommareddy A, Gunna MR, Dokuparthi P, Gottam HB, Botha KK, Parapati DR, Vinod TK (2009) Synlett 1:117
Yusubov MS, Zagulyaeva AA, Zhdankin VV (2009) Chem Eur J 15:11091
Stang PJ, Zhdankin VV (1996) Chem Rev 96:1123
Shim WG, Jung SC, Seo SG, Kim SC (2011) Catal Today 164:500
Khurana JM, Nand B (2010) Can J Chem 88:906
Khurana JM, Agrawal A, Kumar S (2009) J Braz Chem Soc 20:1256
Khurana JM, Kandpal BM (2003) Tetrahedron Lett 44:4909
Khurana JM, Kandpal BM, Chauhan YK (2003) Phosphorus Sulfur Silicon Relat Elem 178:1369
Kikuchi D, Sakaguchi S, Ishii Y (1998) J Org Chem 63:6023
Kajigaeshi S, Nakagawa T, Nagasaki N, Yamasaki H, Fujusaki S (1986) Bull Chem Soc Jpn 59:747
Tomioka H, Oshima K, Nozaki H (1982) Tetrahedron Lett 23:539
Wang YL, Zhao YW, Wang XX, Duan ZF (2002) Synth Commun 32:1781
Metsger L, Bittner S (2000) Tetrahedron 56:1905
Shaabani A, Bazgir A, Abdoli M (2002) Synth Commun 32:675
Shaabani A, Bazgir A, Soleimani K, Salehi P (2003) Synth Commun 33:2935
Shaabani A, Soleimani K, Bazgir A (2004) Synth Commun 34:3303
Shaabani A, Lee DG (2003) Sulfur Lett 26:43
Wasserschield P, Keim W (2000) Angew Chem Int Ed 39:3772
Hajipour AR, Khazdooz L, Ruoho AE (2008) Catal Commun 9:89
Greaves TL, Drummond CJ (2008) Chem Rev 108:206
Guo S, Du Z, Zhang S, Li D, Li Z, Deng Y (2006) Green Chem 8:296
Muzart J (2006) Adv Synth Catal 348:275
Shaabani A, Farhangi E, Rahmati A (2008) Monatsh Chem 139:905
Safari J, Arani NM, Isfahani AR (2011) Asian J Chem 23:495
Kulkarni GC, Karmarkar SN, Kelkar SL, Murzban WS (1988) Tetrahedron 44:5189
Shimakawa Y, Morikawa T, Sakaguchi S (2010) Tetrahedron Lett 51:1786
Rose CA, Gundala S, Cannon SJ, Zeitler K (2011) Synthesis 190
Wang X, Zhang Y (2003) Tetrahedron 59:4201
Jaunin R, Sechaud G (1956) Helv Chim Acta 39:1257
Chen CT, Kao JQ, Salunke SB, Kin YH (2011) Org Lett 13:26
Futami Y, Nishino H, Kurosawa K (1989) Bull Chem Soc Jpn 62:3182
Gregoire B, Carre MC, Caubere P (1986) J Org Chem 51:1419
Jing X, Pan X, Li Z, Shi Y, Yan C (2009) Synth Commun 39:492
Shine HJ, Rangappa P, Marx JN, Shelly DC, Ould-Ely T, Whitmire KH (2005) J Org Chem 70:3877
Syper L (1987) Tetrahedron 43:2853
Sharghi H, Jokar M, Mahboubeh D, Mohammad M, Khalifeh R (2010) Adv Synth Catal 352:3031
Chen JY, Chen SJ, Tang YJ, Mou CY, Tsai FY (2009) J Mol Catal A: Chem 307:88
Marvel CS, Nichols V (1941) J Org Chem 6:296
Cunningham A, Mokal-Parekh V, Wilson C, Woodward S (2004) Org Biomol Chem 2:741
Paakkonen S, Pursiainen J, Lajunen M (2010) Tetrahedron Lett 51:6695
Tucker-Schwartz AK, Garrell RL (2010) Chem Eur J 16:12718
Ali MH, Greene S, Wiggin CJ, Khan S (2006) Synth Commun 36:1761
Maddani M, Prabhu KR (2008) Tetrahedron Lett 49:4526
Xi B, Nevalainen V (2006) Tetrahedron Lett 47:7133
Iinuma M, Moriyama K (2013) Tetrahedron 69:2961
Rolfe A, Probst DA, Volp KA, Omar I, Flynn DL, Hanson PR (2008) J Org Chem 73:8785
Akkilagunta VK, Reddy VP, Kakulapati RR (2010) Synlett 17:2571
Acknowledgments
AL thanks University of Delhi for the award of University Teaching Assistantship and AC thanks C.S.I.R., New Delhi, India for the grant of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khurana, J.M., Lumb, A. & Chaudhary, A. NaBrO3/bmim[HSO4]: a versatile system for the selective oxidation of 1,2-diols, α-hydroxyketones, and alcohols. Monatsh Chem 148, 381–386 (2017). https://doi.org/10.1007/s00706-016-1749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1749-z