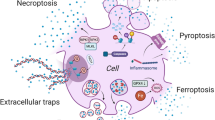
Abstract
Pyroptosis, also known as inflammatory necrosis, is a form of programmed cell death, which is an important natural immune response. Pyroptosis plays a major role in combating pathogenic infections. The mechanism of pyroptosis is distinct from other forms of cell death and is characterized by its dependence on inflammatory caspases (mainly caspases 1, 4, 5, and 11). Activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory vesicles is involved in caspase-1 activation and cleavage, which in turn triggers cleavage and multimerization of multiple gasdermin family members, including gasdermin-D (GSDMD). This further leads to cell perforation and cellular distension, causing cell membrane rupture, resulting in a massive efflux of cell contents, which triggers inflammatory reactions. In recent years, detailed study of viral diseases, has demonstrated that pyroptosis is closely associated with the development of viral diseases. This article focuses on the mechanism of pyroptosis and the connection between pyroptosis and viral infection.


Similar content being viewed by others
Data availability
This is a theoretical study with no experimental data.
References
Verburg SG, Lelievre RM, Westerveld MJ, Inkol JM, Sun YL, Workenhe ST (2022) Viral-mediated activation and inhibition of programmed cell death. Plos Pathog. https://doi.org/10.1371/journal.ppat.1010718
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19(1):107–120. https://doi.org/10.1038/cdd.2011.96
Ketelut-Carneiro N, Fitzgerald KA (2022) Apoptosis, pyroptosis, and necroptosis-oh my! the many ways a cell can die. J Mol Biol. https://doi.org/10.1016/j.jmb.2021.167378
Tang DL, Kang R, Vanden Berghe T, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–364. https://doi.org/10.1038/s41422-019-0164-5
Chen YJ, Smith MR, Thirumalai K, Zychlinsky A (1996) A bacterial invasin induces macrophage apoptosis by binding directly to ICE. Embo J 15(15):3853–3860. https://doi.org/10.1002/j.1460-2075.1996.tb00759.x
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11(12):1136-U1194. https://doi.org/10.1038/ni.1960
Cookson BT, Brennan MA (2001) Pro-inflammatory programmed cell death. Trends Microbiol 9(3):113–114. https://doi.org/10.1016/s0966-842x(00)01936-3
Siegel RM (2006) Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol 6(4):308–317. https://doi.org/10.1038/nri1809
Del Re DP, Amgalan D, Linkermann A, Liu QH, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1763–1817. https://doi.org/10.1152/physrev.00022.2018
Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK (2016) Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165(5):1106–1119. https://doi.org/10.1016/j.cell.2016.04.015
Yazdi AS, Guarda G, D’Ombrain MC, Drexler SK (2010) Inflammatory caspases in innate immunity and inflammation. J Innate Immun 2(3):228–237. https://doi.org/10.1159/000283688
Wang K, Sun Q, Zhong X, Zeng MX, Zeng H, Shi XY, Li ZL, Wang YP, Zhao Q, Shao F et al (2020) Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 180(5):941. https://doi.org/10.1016/j.cell.2020.02.002
Doitsh G, Greene WC (2016) Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe 19(3):280–291. https://doi.org/10.1016/j.chom.2016.02.012
Lei Q, Yi T, Chen C (2018) NF-kappa B-Gasdermin D (GSDMD) Axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monitor 24:6044-6052. https://doi.org/10.12659/Msm.908529
Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75. https://doi.org/10.1111/imr.12534
Liu X, Zhang ZB, Ruan JB, Pan YD, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610):153. https://doi.org/10.1038/nature18629
Fang Y, Tian SW, Pan YT, Li W, Wang QM, Tang Y, Yu T, Wu X, Shi YK, Ma P et al (2020) Pyroptosis: a new frontier in cancer. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2019.109595
Vande Walle L, Lamkanfi M (2016) Pyroptosis. Curr Biol 26(13):R568–R572. https://doi.org/10.1016/j.cub.2016.02.019
Chen S, Mei SH, Luo YJ, Wu H, Zhang JM, Zhu JM (2018) Gasdermin family: a promising therapeutic target for stroke. Transl Stroke Res 9(6):555–563. https://doi.org/10.1007/s12975-018-0666-3
Hu YC, Jiang YY, Li S, Ma XQ, Chen M, Yang R, Wen S, Moynagh PN, Wang BW, Hu G et al (2022) The Gasdermin D N-terminal fragment acts as a negative feedback system to inhibit inflammasome-mediated activation of Caspase-1/11. P Natl Acad Sci USA. https://doi.org/10.1073/pnas.2210809119
Feng SY, Fox D, Man SM (2018) Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol 430(18):3068–3080. https://doi.org/10.1016/j.jmb.2018.07.002
Wang W, Zhang TQ (2018) Caspase-1-mediated pyroptosis of the predominance for driving CD4 T cells death: a nonlocal spatial mathematical model. B Math Biol 80(3):540–582. https://doi.org/10.1007/s11538-017-0389-8
Zhang Z, Shao XH, Jiang N, Mou S, Gu LY, Li S, Lin QS, He YP, Zhang MF, Zhou WY et al (2018) Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. https://doi.org/10.1038/s41419-018-1023-x
Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB (2012) Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med 209(11):1969–1983. https://doi.org/10.1084/jem.20121960
Lupfer C, Kanneganti TD (2013) Unsolved mysteries in NLR biology. Front Immunol. https://doi.org/10.3389/fimmu.2013.00285
Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V (2021) Human NLRP1 is a sensor for double-stranded RNA. Sci (NY, NY) 371(6528):eabd0811. https://doi.org/10.1126/scienceabd0811
Jorgensen I, Miao EA (2015) Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 265(1):130–142. https://doi.org/10.1111/imr.12287
Fernandes-Alnemri T, Yu JW, Datta P, Wu JH, Alnemri ES (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458(7237):509-U505. https://doi.org/10.1038/nature07710
Rathinam VAK, Jiang ZZ, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E et al (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11(5):395–403. https://doi.org/10.1038/ni.1864
Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341(6151):1250–1253. https://doi.org/10.1126/science.1240988
Gurung P, Malireddi RKS, Anand PK, Demon D, Vande Walle L, Liu ZP, Vogel P, Lamkanfi M, Kanneganti TD (2012) Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem 287(41):34474–34483. https://doi.org/10.1074/jbc.M112.401406
Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665. https://doi.org/10.1038/nature15514
Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P (2016) An evolutionary perspective on the necroptotic pathway. Trends Cell Biol 26(10):721–732. https://doi.org/10.1016/j.tcb.2016.06.004
Rex DAB, Prasad TSK, Kandasamy RK (2022) Revisiting regulated cell death responses in viral infections. Int J Mol Sci. https://doi.org/10.3390/ijms23137023
Zitvogel L, Kroemer G (2008) The immune response against dying tumor cells: avoid disaster, achieve cure. Cell Death Differ 15(1):1–2. https://doi.org/10.1038/sj.cdd.4402267
Lupfer C, Malik A, Kanneganti TD (2015) Inflammasome control of viral infection. Curr Opin Virol 12:38–46. https://doi.org/10.1016/j.coviro.2015.02.007
He ZJ, Chen JH, Zhu X, An S, Dong XH, Yu JC, Zhang SH, Wu Y, Li G, Zhang Y et al (2018) NLRP3 inflammasome activation mediates zika virus-associated inflammation. J Infect Dis 217(12):1942–1951. https://doi.org/10.1093/infdis/jiy129
Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S et al (1995) A novel costimulatory factor for gamma-interferon induction found in the livers of mice causes endotoxic-shock. Infect Immun 63(10):3966–3972. https://doi.org/10.1128/Iai.63.10.3966-3972.1995
Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39(6):1003–1018. https://doi.org/10.1016/j.immuni.2013.11.010
Sergerie Y, Rivest S, Boivin G (2007) Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis 196(6):853–860. https://doi.org/10.1086/520094
Jin TC, Perry A, Jiang JS, Smith P, Curry JA, Unterholzner L, Jiang ZZ, Horvath G, Rathinam VA, Johnstone RW et al (2012) Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36(4):561–571. https://doi.org/10.1016/j.immuni.2012.02.014
Man SM, Kanneganti TD (2015) Regulation of inflammasome activation. Immunol Rev 265(1):6–21. https://doi.org/10.1111/imr.12296
Atluri VSR, Pilakka-Kanthikeel S, Garcia G, Jayant RD, Sagar V, Samikkannu T, Yndart A, Nair M (2016) Effect of cocaine on HIV infection and inflammasome gene expression profile in HIV infected macrophages. Sci Rep UK. https://doi.org/10.1038/srep27864
Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin TC, Latz E, Xiao TS et al (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11(11):997-U942. https://doi.org/10.1038/ni.1932
Luan Y, Lengyel P, Liu CJ (2008) p204, a p200 family protein, as a multifunctional regulator of cell proliferation and differentiation. Cytokine Growth F R 19(5–6):357–369. https://doi.org/10.1016/j.cytogfr.2008.11.002
Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9(5):363–375. https://doi.org/10.1016/j.chom.2011.04.008
Dell’Oste V, Gatti D, Giorgio AG, Gariglio M, Landolfo S, De Andrea M (2015) The interferon-inducible DNA-sensor protein IFI16: a key player in the antiviral response. New Microbiol 38(1):5–20
Huang Y, Xu W, Zhou RB (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18(9):2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Lupfer C, Kanneganti TD (2013) The expanding role of NLRs in antiviral immunity. Immunol Rev 255(1):13–24. https://doi.org/10.1111/imr.12089
Sharma D, Kanneganti TD (2016) The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol 213(6):617–629. https://doi.org/10.1083/jcb.201602089
Thomas PG, Dash P, Aldridge JR, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL et al (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30(4):566–575. https://doi.org/10.1016/j.immuni.2009.02.006
Tate MD, Ong JDH, Dowling JK, McAuley JL, Robertson AB, Latz E, Drummond GR, Cooper MA, Hertzog PJ, Mansell A (2016) Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep-Uk. https://doi.org/10.1038/srep27912
Zhao CY, Zhao W (2020) NLRP3 inflammasome-A key player in antiviral responses. Front Immunol. https://doi.org/10.3389/fimmu.2020.00211
Kanneganti TD (2010) Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol 10(10):688–698. https://doi.org/10.1038/nri2851
Keller M, Ruegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132(5):818–831. https://doi.org/10.1016/j.cell.2007.12.040
Li JN, Hu L, Liu YY, Huang L, Mu Y, Cai XH, Weng CJ (2015) DDX19A senses viral RNA and mediates NLRP3-dependent inflammasome activation. J Immunol 195(12):5732–5749. https://doi.org/10.4049/jimmunol.1501606
Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U et al (2012) Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. https://doi.org/10.1038/ncomms2339
Dominic A, Le NT, Takahashi M (2022) Loop between NLRP3 inflammasome and reactive oxygen species. Antioxid Redox Sign 36(10):784–796. https://doi.org/10.1089/ars.2020.8257
Wang C, Yang RY, Yang FX, Han Y, Ren YJ, Xiong XB, Wang XY, Bi YD, Li LJ, Qiu Y et al (2022) Echovirus 11 infection induces pyroptotic cell death by facilitating NLRP3 inflammasome activation. Plos Pathog. https://doi.org/10.1371/journal.ppat.1010787
Triantafilou K, Triantafilou M (2014) Ion flux in the lung: virus-induced inflammasome activation. Trends Microbiol 22(10):580–588. https://doi.org/10.1016/j.tim.2014.06.002
Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JPY, Bose S (2012) TLR2/MyD88/NF-kappa B pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. Plos One. https://doi.org/10.1371/journal.pone.0029695
Jorgensen I, Rayamajhi M, Miao EA (2017) Programmed cell death as a defence against infection. Nat Rev Immunol 17(3):151–164. https://doi.org/10.1038/nri.2016.147
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426. https://doi.org/10.1016/S1097-2765(02)00599-3
Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ et al (2012) NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37(6):1009–1023. https://doi.org/10.1016/j.immuni.2012.08.027
Xia P, Xing XD, Yang CX, Liao XJ, Liu FH, Huang HH, Zhang C, Song JW, Jiao YM, Shi M et al (2022) Activation-induced pyroptosis contributes to the loss of MAIT cells in chronic HIV-1 infected patients. Military Med Res. https://doi.org/10.1186/s40779-022-00384-1
Monroe KM, Yang ZY, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC (2014) IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343(6169):428–432. https://doi.org/10.1126/science.1243640
Vijayan KKV, Karthigeyan KP, Tripathi SP, Hanna LE (2017) Pathophysiology of CD4+T-cell depletion in HIV-1 and HIV-2 infections. Front Immunol. https://doi.org/10.3389/fimmu.2017.00580
Finkel TH, Tudorwilliams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A (1995) Apoptosis occurs predominantly in bystander cells and not in productively infected-cells of hiv-infected and siv-infected lymph-nodes. Nat Med 1(2):129–134. https://doi.org/10.1038/nm0295-129
Galloway NLK, Doitsh G, Monroe KM, Yang ZY, Munoz-Arias I, Levy DN, Greene WC (2015) Cell-to-cell transmission of HIV-1 Is required to trigger pyroptotic death of lymphoid-tissue-derived CD4 T cells. Cell Rep 12(10):1555–1563. https://doi.org/10.1016/j.celrep.2015.08.011
Doitsh G, Galloway NLK, Geng X, Yang ZY, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I et al (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505(7484):509. https://doi.org/10.1038/nature12940
Doitsh G, Galloway NLK, Geng X, Yang ZY, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I et al (2017) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection (vol 505, pg 509, 2014). Nature 544(7648):124–124. https://doi.org/10.1038/nature22066
Paoletti A, Allouch A, Caillet M, Saidi H, Subra F, Nardacci R, Wu QJ, Muradova Z, Voisin L, Raza SQ et al (2019) HIV-1 envelope overcomes NLRP3-mediated inhibition of F-actin polymerization for viral entry. Cell Rep 28(13):3381. https://doi.org/10.1016/j.celrep.2019.02.095
Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59(3):898–910. https://doi.org/10.1002/hep.26592
Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, Guo QL, Gao WY, Wang XZ, Li D (2020) Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res 69(7):683–696. https://doi.org/10.1007/s00011-020-01351-z
Tacke F (2017) Targeting hepatic macrophages to treat liver diseases. J Hepatol 66(6):1300–1312. https://doi.org/10.1016/j.jhep.2017.02.026
Yu X, Lan PX, Hou XB, Han QJ, Lu N, Li T, Jiao CW, Zhang J, Zhang C, Tian ZG (2017) HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1 beta production via suppressing the NF-kappa B pathway and ROS production. J Hepatol 66(4):693–702. https://doi.org/10.1016/j.jhep.2016.12.018
Tan TY, Chu JJH (2013) Dengue virus-infected human monocytes trigger late activation of caspase-1, which mediates pro-inflammatory IL-1 beta secretion and pyroptosis. J Gen Virol 94:2215–2220. https://doi.org/10.1099/vir.0.055277-0
Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, Tsai IS, Li L, Hsieh SL (2013) CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 121(1):95–106. https://doi.org/10.1182/blood-2012-05-430090
Suwanmanee S, Luplertlop N (2017) Immunopathogenesis of dengue virus-induced redundant cell death: apoptosis and pyroptosis. Viral Immunol 30(1):13–19. https://doi.org/10.1089/vim.2016.0092
Kuriakose T, Kanneganti TD (2017) Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol 86:56–64. https://doi.org/10.1016/j.molimm.2017.01.023
Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza a virus through recognition of viral RNA. Immunity 30(4):556–565. https://doi.org/10.1016/j.immuni.2009.02.005
McAuley JL, Tate MD, MacKenzie-Kludas CJ, Pinar A, Zeng WG, Stutz A, Latz E, Brown LE, Mansell A (2013) Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. Plos Pathog. https://doi.org/10.1371/journal.ppat.1003392
Cheong WC, Kang HR, Yoon H, Kang SJ, Ting JPY, Song MJ (2015) Influenza A Virus NS1 Protein Inhibits the NLRP3 Inflammasome. PLoS One. https://doi.org/10.1371/journal.pone.0126456
Weiss SR, Navas-Martin S (2005) Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol R 69(4):635. https://doi.org/10.1128/Mmbr.69.4.635-664.2005
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9(1):221–236. https://doi.org/10.1080/22221751.2020.1719902
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Speciality HA (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannata A, Pillappa R, Sinagra G, Nana-Sinkam P, Sime P et al (2021) Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res 70(1):7–10. https://doi.org/10.1007/s00011-020-01413-2
Rodrigues TS, de Sa KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Goncalves AV, Perucello DB, Andrade WA, Castro R et al (2021) Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. https://doi.org/10.1084/jem.20201707
Zeng JX, Xie XC, Feng XL, Xu L, Han JB, Yu DD, Zou QC, Liu QJ, Li XH, Ma GQ et al (2022) Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine. https://doi.org/10.1016/j.ebiom.2021.103803
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB et al (2019) MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 15(6):556. https://doi.org/10.1038/s41589-019-0277-7
Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias SDG, Fintelman-Rodrigues N, Sacramento CQ, Mattos M, de Freitas CS, Temerozo JR, Teixeira L et al (2021) SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes (vol 7, 43, 2021). Cell Death Discov. https://doi.org/10.1038/s41420-021-00477-1
Ma J, Zhu FR, Zhao M, Shao F, Yu D, Ma JW, Zhang XS, Li WT, Qian Y, Zhang Y, et al. (2021) SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking Gasdermin D cleavage. Embo J. https://doi.org/10.15252/embj.2021108249
Huang Y, Liu LL, Ma D, Liao Y, Lu YY, Huang HY, Qin WQ, Liu XL, Fang F (2017) Human cytomegalovirus triggers the assembly of AIM2 inflammasome in THP-1-derived macrophages. J Med Virol 89(12):2188–2195. https://doi.org/10.1002/jmv.24846
Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B (2013) Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol 87(15):8606–8623. https://doi.org/10.1128/Jvi.00805-13
Johnson KE, Chikoti L, Chandran B (2013) Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87(9):5005–5018. https://doi.org/10.1128/Jvi.00082-13
Wang JL, Alexander J, Wiebe M, Jones C (2014) Bovine herpesvirus 1 productive infection stimulates inflammasome formation and caspase 1 activity. Virus Res 185:72–76. https://doi.org/10.1016/j.virusres.2014.03.006
Zhang M, Covar J, Zhang NY, Chen W, Marshall B, Mo J, Atherton SS (2013) Virus spread and immune response following anterior chamber inoculation of HSV-1 Lacking the Beclin-binding domain (BBD). J Neuroimmunol 260(1–2):82–91. https://doi.org/10.1016/j.jneuroim.2013.03.013
Looi CK, Hii LW, Chung FFL, Mai CW, Lim WM, Leong CO (2021) Roles of inflammasomes in epstein-barr virus-associated nasopharyngeal cancer. Cancers. https://doi.org/10.3390/cancers13081786
Koraka P, Martina BEE, Smreczak M, Orlowska A, Marzec A, Trebas P, Roose JM, Begeman L, Gerhauser I, Wohlsein P et al (2019) Inhibition of caspase-1 prolongs survival of mice infected with rabies virus. Vaccine 37(33):4681–4685. https://doi.org/10.1016/j.vaccine.2018.04.002
Lei XB, Zhang ZZ, Xiao X, Qi JL, He B, Wang JW (2017) Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J Virol. https://doi.org/10.1128/JVI.01069-17
Zhu S, Ding SY, Wang PH, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li HB, Wang G et al (2017) Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546(7660):667. https://doi.org/10.1038/nature22967
Acknowledgments
The authors would like to apologize to the many researchers who have contributed to this area of research but have not been cited in this review due to space limitations.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MH302 [Bing Luo]; ZR2020MC020 [Yan Zhang]).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing—original draft preparation, Zhen Zhao; writing—review and editing, Yan Zhang and Bing Luo. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Handling Editor: Sheela Ramamoorthy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z., Zhang, Y. & Luo, B. The role of pyroptosis in viral infection. Arch Virol 169, 69 (2024). https://doi.org/10.1007/s00705-024-05978-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-024-05978-9