Abstract
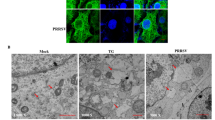
Porcine circovirus type 2 (PCV2) is the essential infectious agent causing porcine circovirus-associated disease (PCVD) in pigs and one of the important viruses that severely jeopardize the swine husbandry industry. PCV2 elicits the unfolded protein response (UPR) via activation of the PERK pathway, and its capsid protein (Cap) has also been found to induce UPR with subsequent activation of apoptosis. The open reading frame 5 (ORF5) protein is a recently discovered non-structural protein, and its function in PCV2 pathogenesis remains unknown. The aim of this study was to determine whether the PCV2 ORF5 protein could induce endoplasmic reticulum stress (ERS) and UPR in porcine alveolar macrophages (PAMs). pEGFP-tagged ORF5 protein was transiently overexpressed in PAMs. Transmission electron microscopy (TEM) was employed to examine changes in ER morphology, and quantitative real-time PCR and western blotting analysis were used to measure UPR-related cell signaling alterations. We found that the ORF5 protein triggers swelling and degranulation of the ER and upregulates the expression of ERS markers. Further experiments demonstrated that the PCV2 ORF5 protein induces ERS and UPR via the PERK (RNA-activated protein kinase-like endoplasmic reticulum kinase), ATF6 (activating transcription factor 6) and IRE1 (inositol requiring enzyme 1) signaling pathways. Together with previous studies, we provide new information on the ERS-UPR induced by the PCV2 ORF5 protein.





Similar content being viewed by others
References
Rose N, Opriessnig T, Grasland B, Jestin A (2012) Epidemiology and transmission of porcine circovirus type 2 (PCV2). Virus Res 164(1–2):78–89. https://doi.org/10.1016/j.virusres.2011.12.002
Segales J, Kekarainen T, Cortey M (2013) The natural history of porcine circovirus type 2: from an inoffensive virus to a devastating swine disease? Vet Microbiol 165(1–2):13–20. https://doi.org/10.1016/j.vetmic.2012.12.033
Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V (2009) Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med 23(6):1151–1163. https://doi.org/10.1111/j.1939-1676.2009.0389.x
Grau-Roma L, Fraile L, Segales J (2011) Recent advances in the epidemiology, diagnosis and control of diseases caused by porcine circovirus type 2. Vet J 187(1):23–32. https://doi.org/10.1016/j.tvjl.2010.01.018
Tomas A, Fernandes LT, Valero O, Segales J (2008) A meta-analysis on experimental infections with porcine circovirus type 2 (PCV2). Vet Microbiol 132(3–4):260–273. https://doi.org/10.1016/j.vetmic.2008.05.023
Hamel AL, Lin LL, Nayar GP (1998) Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J Virol 72(6):5262–5267
Guo LJ, Lu YH, Wei YW, Huang LP, Liu CM (2010) Porcine circovirus type 2 (PCV2): genetic variation and newly emerging genotypes in China. Virol J 7:273. https://doi.org/10.1186/1743-422X-7-273
Lv QZ, Guo KK, Zhang YM (2014) Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes 49(1):1–10. https://doi.org/10.1007/s11262-014-1099-z
Li D, Wang J, Xu S, Cai S, Ao C, Fang L, Xiao S, Chen H, Jiang Y (2018) Identification and functional analysis of the novel ORF6 protein of porcine circovirus type 2 in vitro. Vet Res Commun 42(1):1–10. https://doi.org/10.1007/s11259-017-9702-0
Cheung AK (2003) The essential and nonessential transcription units for viral protein synthesis and DNA replication of porcine circovirus type 2. Virology 313(2):452–459
Mankertz A, Mueller B, Steinfeldt T, Schmitt C, Finsterbusch T (2003) New reporter gene-based replication assay reveals exchangeability of replication factors of porcine circovirus types 1 and 2. J Virol 77(18):9885–9893
Fort M, Sibila M, Nofrarias M, Perez-Martin E, Olvera A, Mateu E, Segales J (2010) Porcine circovirus type 2 (PCV2) Cap and Rep proteins are involved in the development of cell-mediated immunity upon PCV2 infection. Vet Immunol Immunopathol 137(3–4):226–234. https://doi.org/10.1016/j.vetimm.2010.05.013
Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS (2000) Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 81(Pt 9):2281–2287. https://doi.org/10.1099/0022-1317-81-9-2281
Liu J, Chen I, Kwang J (2005) Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J Virol 79(13):8262–8274. https://doi.org/10.1128/JVI.79.13.8262-8274.2005
He J, Cao J, Zhou N, Jin Y, Wu J, Zhou J (2013) Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J Virol 87(3):1420–1429. https://doi.org/10.1128/JVI.01443-12
Lv Q, Guo K, Wang T, Zhang C, Zhang Y (2015) Porcine circovirus type 2 ORF4 protein binds heavy chain ferritin. J Biosci 40(3):477–485
Lv Q, Guo K, Zhang G, Zhang Y (2016) The ORF4 protein of porcine circovirus type 2 antagonizes apoptosis by stabilizing the concentration of ferritin heavy chain through physical interaction. J Gen Virol 97(7):1636–1646. https://doi.org/10.1099/jgv.0.000472
Kitamura M (2008) Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol 295(2):F323–F334. https://doi.org/10.1152/ajprenal.00050.2008
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102. https://doi.org/10.1038/nrm3270
Li S, Kong L, Yu X (2015) The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol 41(2):150–164. https://doi.org/10.3109/1040841X.2013.813899
Hetz C, Chevet E, Oakes SA (2015) Proteostasis control by the unfolded protein response. Nat Cell Biol 17(7):829–838. https://doi.org/10.1038/ncb3184
Zhou YS, Gu YX, Qi BZ, Zhang YK, Li XL, Fang WH (2017) Porcine circovirus type 2 capsid protein induces unfolded protein response with subsequent activation of apoptosis. J Zhejiang Univ Sci B 18(4):316–323. https://doi.org/10.1631/jzus.B1600208
Lv Q, Guo K, Xu H, Wang T, Zhang Y (2015) Identification of putative ORF5 protein of porcine circovirus type 2 and functional analysis of gfp-fused ORF5 protein. PLoS One 10(6):e0127859. https://doi.org/10.1371/journal.pone.0127859
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5(5):897–904
Tsukumo Y, Tsukahara S, Furuno A, Iemura S, Natsume T, Tomida A (2014) TBL2 is a novel PERK-binding protein that modulates stress-signaling and cell survival during endoplasmic reticulum stress. PLoS One 9(11):e112761. https://doi.org/10.1371/journal.pone.0112761
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633
Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153(5):1011–1022
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Investig 110(10):1383–1388. https://doi.org/10.1172/JCI16784
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6(6):1355–1364
Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3(1):99–111
Shen J, Prywes R (2004) Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem 279(41):43046–43051. https://doi.org/10.1074/jbc.M408466200
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20(18):6755–6767
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366(Pt 2):585–594. https://doi.org/10.1042/BJ20020391
Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258
Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T (2000) A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol 2(7):379–384. https://doi.org/10.1038/35017001
Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4(2):265–271
Trujillo-Alonso V, Maruri-Avidal L, Arias CF, Lopez S (2011) Rotavirus infection induces the unfolded protein response of the cell and controls it through the nonstructural protein NSP3. J Virol 85(23):12594–12604. https://doi.org/10.1128/JVI.05620-11
Hassan IH, Zhang MS, Powers LS, Shao JQ, Baltrusaitis J, Rutkowski DT, Legge K, Monick MM (2012) Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J Biol Chem 287(7):4679–4689. https://doi.org/10.1074/jbc.M111.284695
Zhou Y, Qi B, Gu Y, Xu F, Du H, Li X, Fang W (2016) Porcine circovirus 2 deploys PERK pathway and GRP78 for its enhanced replication in PK-15 cells. Viruses. https://doi.org/10.3390/v8020056
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35(4):373–381. https://doi.org/10.1016/j.ymeth.2004.10.010
Harding HP, Calfon M, Urano F, Novoa I, Ron D (2002) Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol 18:575–599. https://doi.org/10.1146/annurev.cellbio.18.011402.160624
Kobylewski SE, Henderson KA, Yamada KE, Eckhert CD (2017) Activation of the EIF2alpha/ATF4 and ATF6 pathways in DU-145 cells by boric acid at the concentration reported in men at the US mean boron intake. Biol Trace Element Res 176(2):278–293. https://doi.org/10.1007/s12011-016-0824-y
Michalak M, Robert Parker JM, Opas M (2002) Ca2 + signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32(5–6):269–278
Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ (2006) The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA 103(39):14343–14348. https://doi.org/10.1073/pnas.0606480103
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13(3):365–376. https://doi.org/10.1016/j.devcel.2007.07.018
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397(6716):271–274. https://doi.org/10.1038/16729
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101(31):11269–11274. https://doi.org/10.1073/pnas.0400541101
Zhang F, Moon A, Childs K, Goodbourn S, Dixon LK (2010) The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J Virol 84(20):10681–10689. https://doi.org/10.1128/JVI.01027-10
Cheng G, Feng Z, He B (2005) Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)3.45 protein. J Virol 79(3):1379–1388. https://doi.org/10.1128/jvi.79.3.1379-1388.2005
Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275(35):27013–27020. https://doi.org/10.1074/jbc.M003322200
Li B, Gao B, Ye L, Han X, Wang W, Kong L, Fang X, Zeng Y, Zheng H, Li S, Wu Z, Ye L (2007) Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res 124(1–2):44–49. https://doi.org/10.1016/j.virusres.2006.09.011
Ambrose RL, Mackenzie JM (2013) ATF6 signaling is required for efficient West Nile virus replication by promoting cell survival and inhibition of innate immune responses. J Virol 87(4):2206–2214. https://doi.org/10.1128/JVI.02097-12
Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW (2007) Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem 282(10):7024–7034. https://doi.org/10.1074/jbc.M609490200
Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci 122(Pt 10):1626–1636. https://doi.org/10.1242/jcs.045625
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27(1):53–66. https://doi.org/10.1016/j.molcel.2007.06.011
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Tardif KD, Mori K, Kaufman RJ, Siddiqui A (2004) Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem 279(17):17158–17164. https://doi.org/10.1074/jbc.M312144200
Perera N, Miller JL, Zitzmann N (2017) The role of the unfolded protein response in dengue virus pathogenesis. Cell Microbiol 19(5):5. https://doi.org/10.1111/cmi.12734
Funding
This work was supported by grants from the National Natural Science Foundation of China (Project No. 31672580).
Author information
Authors and Affiliations
Contributions
YLOY, KKG, and YMZ designed the experiments. YLOY, LX, JML and YFH carried out the experiments, collected data, and wrote this manuscript. KKG and YMZ contributed to critical revision of the manuscript. ZXF, PPX, YFJ, RL and MMW contributed to acquisition of data and critical revision of the manuscript. KKG contributed to the study concept and design, obtaining funding, study supervision, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Zhenhai Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ouyang, Y., Xu, L., Lv, J. et al. Porcine circovirus type 2 ORF5 protein induces endoplasmic reticulum stress and unfolded protein response in porcine alveolar macrophages. Arch Virol 164, 1323–1334 (2019). https://doi.org/10.1007/s00705-019-04185-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04185-1