Abstract
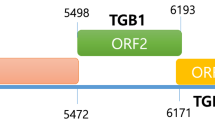
New genomic sequence data were acquired for the Acipenser iridovirus-European (AcIV-E), a virus whose complete genome and classification still remain to be elucidated. Here, we obtained the first full-length Major capsid protein (MCP) gene sequence for AcIV-E, as well as two additional open reading frames (ORFs) adjacent to the MCP gene. BLAST searches of the first ORF (α) resulted in no match to any gene or protein in the public databases. The other ORF (β) was identified as a subunit of a replication factor C (RFC), known to function as a clamp loader in eukaryotes, archae and some viruses. The presence of similar RFC genes was confirmed in two distinct, yet related, viruses, the white sturgeon iridovirus and a European variant of Namao virus. The existence of an RFC gene in AcIV-E suggests a genome size larger than that of other classifiable members of the family Iridoviridae along with a mode of replication involving an interaction between a clamp loader and a proliferating nuclear cell antigen. Sequencing and comparison of the full-length RFC gene from various sturgeon samples infected with AcIV-E revealed two distinct clusters of sequences within one particular sample in which the coexistence of two lineages had previously been predicted based on analysis of the partial MCP gene sequence. These genetic data provide further evidence of the circulation of at least two concurrent AcIV-E lineages, sometimes co-infecting cultured European sturgeon.





Similar content being viewed by others
References
Bigarré L, Lesne M, Lautraite A, Chesneau V, Leroux A, Jamin M, Boitard PM, Toffan A, Prearo M, Labrut S, Daniel P (2017) Molecular identification of iridoviruses infecting various sturgeon species in Europe. J Fish Dis 40:105–118
Ciulli S, Volpe E, Sirri R, Passalacqua PL, Cesa Bianchi F, Serratore P, Mandrioli L (2016) Outbreak of mortality in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) sturgeons associated with sturgeon nucleo-cytoplasmatic large DNA virus. Vet Microbiol 191:27–34
Clouthier SC, Vanwalleghem E, Copeland S, Klassen C, Hobbs G, Nielsen O, Anderson ED (2013) A new species of nucleo-cytoplasmic large DNA virus (NCLDV) associated with mortalities in Manitoba lake sturgeon Acipenser fulvescens. Dis Aquat Organ 102:195–209
Clouthier SC, VanWalleghem E, Anderson ED (2015) Sturgeon nucleo-cytoplasmic large DNA virus phylogeny and PCR tests. Dis Aquat Organ 117:93–106
Clouthier SC, Breyta R, Kurath G, Anderson E (2017) Sturgeon nucleo-cytoplasmic large DNA virus phylogeny. In: 10th international symposium on lower vertebrates, 4–7 June, Budapest
Clouthier SC, McClure C, Schroeder T, Desai M, Hawley L, Khatkar S, Lindsay M, Lowe G, Richard J, Anderson ED (2017) Diagnostic validation of three test methods for detection of cyprinid herpesvirus 3 (CyHV-3). Dis Aquat Organ 123:101–122
Hedrick RP, Groff JM, McDowell T, Wingfield WH (1990) An iridovirus infection of the integument of the white sturgeon Acipenser transmontanus. Dis Aquat Organ 8:39–44
Hedrick RP, McDowell TS, Groff JM, Yun S, Wingfield WH (1992) Isolation and some properties of an iridovirus-like agent from white sturgeon Acipenser transmontanus. Dis Aquat Organ 12:75–81
Henneke G, Gueguen Y, Flament D, Azam P, Querellou J, Dietrich J, Hubscher U, Raffin JP (2002) Replication factor C from the hyperthermophilic archaeon Pyrococcus abyssi does not need ATP hydrolysis for clamp-loading and contains a functionally conserved RFC PCNA-binding domain. J Mol Biol 323:795–810
Kazlauskas D, Venclovas C (2011) Computational analysis of DNA replicases in double-stranded DNA viruses: relationship with the genome size. Nucleic Acids Res 39:8291–8305
Kazlauskas D, Krupovic M, Venclovas C (2016) The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes. Nucleic Acids Res 44:4551–4564
Kelch BA (2016) Review: The lord of the rings: structure and mechanism of the sliding clamp loader. Biopolymers 105:532–546
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kurobe T, Kwak KT, MacConnell E, McDowell TS, Mardones FO, Hedrick RP (2010) Development of PCR assays to detect iridovirus infections among captive and wild populations of Missouri River sturgeon. Dis Aquat Organ 93:31–42
Kurobe T, MacConnell E, Hudson C, McDowell TS, Mardones FO, Hedrick RP (2011) Iridovirus infections among Missouri River sturgeon: initial characterization, transmission, and evidence for establishment of a carrier state. J Aquat Anim Health 23:9–18
Kwak KT, Gardner IA, Farver TB, Hedrick RP (2006) Rapid detection of white sturgeon iridovirus (WSIV) using a polymerase chain reaction (PCR) assay. Aquaculture 254:92–101
Oliveira GP, Lima MT, Arantes TS, Assis FL, Rodrigues RAL, da Fonseca FG, Bonjardim CA, Kroon EG, Colson P, La Scola B, Abrahao JS (2017) The investigation of promoter sequences of Marseilleviruses highlights a remarkable abundance of the AAATATTT motif in intergenic regions. J Virol 91:1–10
Seybert A, Singleton MR, Cook N, Hall DR, Wigley DB (2006) Communication between subunits within an archaeal clamp-loader complex. EMBO J 25:2209–2218
Suhre K, Audic S, Claverie JM (2005) Mimivirus gene promoters exhibit an unprecedented conservation among all eukaryotes. Proc Natl Acad Sci USA 102:14689–14693
Sun Q, Tsurimoto T, Juillard F, Li L, Li S, De Leon Vazquez E, Chen S, Kaye K (2014) Kaposi’s sarcoma-associated herpesvirus LANA recruits the DNA polymerase clamp loader to mediate efficient replication and virus persistence. Proc Natl Acad Sci USA 111:11816–11821
Triglia T (2000) Inverse PCR (IPCR) for obtaining promoter sequence. Methods Mol Biol 130:79–83
Waltzek T (2017) Overview of the nucleo-cytoplasmic large DNA viruses (NCLDVs). In: 10th international symposium on lower vertebrates, 4–7 June, Budapest
Waltzek TB, Miller DL, Gray MJ, Drecktrah B, Briggler JT, MacConnell B, Hudson C, Hopper L, Friary J, Yun SC, Malm KV, Weber ES, Hedrick RP (2014) New disease records for hatchery-reared sturgeon. I. Expansion of frog virus 3 host range into Scaphirhynchus albus. Dis Aquat Organ 111:219–227
Zuccola HJ, Filman DJ, Coen DM, Hogle JM (2000) The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol Cell 5:267–278
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study (ACIVIR2 project) was funded by the European Regional Development Fund (FEDER 160710), the Nouvelle Aquitaine regional council and partner fish farmers.
Conflict of interest
B Debeuf and V. Chesneau work for private companies cited in the authors’ list. The other authors declare that there are no competing interests regarding the publication of this paper.
Ethical approval
The samples originated from moribund farmed fish, not submitted to experimentations and euthanized in accordance with animal welfare and ethics.
Additional information
Handling Editor: Chan-Shing Lin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pallandre, L., Lesne, M., de Boisséson, C. et al. Acipenser iridovirus-European encodes a replication factor C (RFC) sub-unit. Arch Virol 163, 2985–2995 (2018). https://doi.org/10.1007/s00705-018-3963-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3963-y