Abstract
Potato virus Y (PVY) infection has been a global challenge for potato production and the leading cause of downgrading and rejection of seed crops for certification. Accurate and timely diagnosis is a key for effective disease control. Here, we have optimized a reverse transcription loop-mediated amplification (RT-LAMP) assay to differentiate the PVY O and N serotypes. The RT-LAMP assay is based on isothermal autocyclic strand displacement during DNA synthesis. The high specificity of this method relies heavily on the primer sets designed for the amplification of the targeted regions. We designed specific primer sets targeting a region within the coat protein gene that contains nucleotide signatures typical for O and N coat protein types, and these primers differ in their annealing temperature. Combining this assay with total RNA extraction by magnetic capture, we have established a highly sensitive, simplified and shortened RT-LAMP procedure as an alternative to conventional nucleic acid assays for diagnosis. This optimized procedure for virus detection may be used as a preliminary test for identifying the viral serotype prior to investing time and effort in multiplex RT-PCR tests when a specific strain is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato virus Y (PVY) is the type member of the genus Potyvirus, family Potyviridae [9]. It has recently been listed among the 10 most important plant viruses in the world [12] and recognized as the major virus infecting potato [8]. A major challenge in the control of PVY infection is the continual emergence of new recombinants derived mostly from the ordinary PVYO strain and the necrotic PVYN strain [2, 5, 7]. At least nine PVY strains are currently known and differ at the biological, serological, and molecular levels but can be grouped into two serotypes: the O serotype, which includes PVYO, PVYN:O, PVYN-Wi, and PVYC, and the N serotype, which includes PVYN, PVYE, PVYZ, PVYNA-N and PVYNE11 [8]. Due to their economic importance [2, 8], the identification of PVY isolates by strain, or at least by serotype, is needed. Many procedures for the detection of PVY in potato leaves and tubers have been reported [3, 6, 13,14,15,16]. Due to their easy adaptation to automation, their high specificity, and their ability to detect viral strains in a single reaction, real-time-RT-PCR-based assays appear to be ideal successors of the double sandwich enzyme-linked immunosorbent assay (DAS-ELISA) that is routinely used in the grow-out test of dormant tubers for the diagnosis of potato viruses for seed certification [3]. However, such methods have not yet been adapted for routine diagnostic schemes.
Here, we describe a streamlined magnetic capture version of the reverse transcription loop-mediated amplification (MC-RT-LAMP) assay that we optimized for fast detection and discrimination of PVY by their serotypes. To contrast this assay with previously reported RT-LAMP variants for the detection of PVY [1, 10, 11], we designed a primer set that amplified a conserved region within the coat protein gene that contains two single and two double nucleotide signatures typical for O and N coat protein types, which differ in their annealing temperatures in the RT-LAMP assay depending on the serotype of the virus tested. This allowed us to rapidly distinguish the PVY O and PVY N types in one reaction. In addition, the optimization of the RNA isolation step by magnetic capture, both from potato leaves and tubers, shortened the procedure while retaining its high sensitivity. The present study offers a fast alternative to conventional nucleic-acid-based assays and to the routinely used DAS-ELISA for grow-out tests, which can be time consuming, for the direct identification of PVY isolates.
Materials and methods
Source of plants and viruses
Potato tubers (cultivars Liwia, Nysa and Wilga) infected with PVY isolates 12/94, N Nysa, Wi and LW were obtained from the Młochów Research Center of the Plant Breeding and Acclimatization Institute – National Research Institute. The PVY isolates PV-0403, PV-0410, PV-0348, PV-0345, and PV-0343 were purchased from the plant virus collection maintained by the Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures. Leaf samples infected with PVY isolates Bonin 1-3 were collected in local experimental fields. Strain characteristics and GenBank accession numbers (where available) are shown in Table 1.
Potato tubers of cultivars Osa, Giewont, Leona, and Pungo infected with potato leafroll virus (PLRV), potato virus M (PVM), potato virus S (PVS), and potato virus X (PVX), respectively, and virus-free tubers of cultivar Sagitta were obtained from the Laboratory of Genetic Resources and In Vitro Cultures of the Department of Potato Protection and Seed Science in Bonin, Plant Breeding and Acclimatization Institute National Research Institute.
Unless indicated otherwise, PVY isolate 12/94 (PVYNTN) was used in all experiments.
RNA isolation
Total RNA was extracted using different methods as indicated. The initial sap was extracted from approximately 100-200 mg of potato leaf or tuber tissue.
Silica capture procedure
Total nucleic acid was purified using the silica capture procedure as described by Zacharzewska et al. [16]. Nine hundred microliters of L6 buffer (5.25 M guanidinium thiocyanate, 50 mM Tris-HCl, pH 6.4, 20 mM EDTA, and 1.3% Triton X-100) was added to 50 µl of silica beads and mixed with 100 µl of undiluted plant sap. After a 10-min incubation at room temperature with gentle shaking, the samples were centrifuged at 12,000g. The pellet was next washed twice with 1 ml of L2 buffer (5.25 M guanidinium thiocyanate, 50 mM Tris-HCl, pH 6.4), twice with ethanol, and once with acetone. The final pellet was dried, resuspended in 50 µl of RNase-free water, and incubated for 10 min at 56 °C. The samples were next quickly centrifuged, and the supernatants containing total nucleic acids were collected.
Manufacturer’s magnetic capture procedure
Total RNA was purified from 100 µl of undiluted sap using a Novabeads Total RNA Purification Kit (Novazym Polska s. c.) according to the manufacturer’s protocol.
Modified magnetic capture procedure
All steps and buffers were as described as above for the silica capture method except that the silica beads were replaced with 25 µl of magnetic particle suspensions from the Novabeads Total RNA Purification Kit (Novazym Polska s. c.). All centrifugation steps were replaced by the use of a magnetic stand.
Shortened magnetic capture procedure
This is a shortened version of the modified magnetic capture procedure. One hundred microliters of undiluted sap was added to 25 µl of Novabeads magnetic particles (Novazym Polska s. c.), which were resuspended in L6 buffer. The samples were incubated at room temperature with gentle shaking for 10 min. Next, the magnetic particles were captured on a magnetic stand, and the supernatants were removed. The beads were washed with L2 buffer and then with 70% ethanol. The beads were finally resuspended in 50 µl of RNase-free water and incubated for 5 min at 56 °C prior to collection of the supernatants.
For RT-LAMP and real-time RT-PCR, total nucleic acid preparations were made without prior DNAse treatment. Each RNA isolation was conducted with at least two replicates and was repeated independently at least three times.
RT-LAMP assay
Different primer sets were tested in this study, including the N set developed by Nie [10], the Y5 set developed by Przewodowska et al. [11], and a new Y4 primer set that we designed using LAMP Designer software (Premier Biosoft) as described by Przewodowska et al. [11] (Table 2). All primer sets consisted of four (N set) or six (Y4 and Y5 sets) primers and targeted different regions of the PVY coat protein gene (Fig. 1). The RT-LAMP reaction mixture (10 μl) contained 0.375 µM each of outer primer (F3, B3), 1.5 µM each of inner primer (FIP, BIP), 0.75 µM each of loop primer (LF, LB), 1X Isothermal Master Mix containing proprietary fluorescent dye (Novazym Polska sc.), 0.25 U of AMV reverse transcriptase (Novazym Polska s. c.), and 100 pg of total RNA. The amplification was performed either with a Genie II Ultra rapid amplification instrument (OptiGene Ltd.) or with a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Ltd). With the OptiGene instrument, the assay was conducted at 65 °C for 30 min. The annealing temperature (Ta) of the amplified products was analyzed in a slow annealing step (0.05 °C/s) for 5 min, starting at 95 °C and ending at 80 °C, with monitoring of the fluorescence. The thermal profile with the Bio-Rad instrument included 60 cycles of 30 s at 65 °C. The amplification was followed by the analysis of the melting temperature (65 °C to 98 °C, 0.5 °C/s).
Alignment of PVY coat protein sequences with the positioning of the primer sets. The open reading frames of the coat protein (cp) genes of 50 PVY isolates representing O and N types were aligned, and a phylogenetic tree was built (shown in blue) using default setting in Genious Pro 5.0.4 software (Biomatters Ltd.). The annotated base positions 1-801 corresponds to bp 8480-9280 in the genome of PVY isolate N Nysa (FJ666337). The isolate names are followed by their corresponding accession numbers. Non-potato-infecting isolate Chile 3 was used as an out-group. The consensus sequence represents bases matching at least 75% of the aligned sequences. Only disagreements to the consensus are shown as color-coded. Nucleotides in the consensus sequence are color-coded as follows: red, A; yellow, G; blue, C; green, U; gray, ambiguous (R, Y, M). The positions of the RT-LAMP primer sets (Y4, Y5 and N), the real-time RT-PCR primers (FP, RP), and the probe (Pro) are indicated under the alignment. F1 corresponds to the sequence complementary to the F1c region of the FIP primer. B2c corresponds to the sequence complementary to the B2 region of the BIP primer. LFc and B3c correspond to sequences complementary to the LF and B3 primers. The direction of the arrows indicates the 5′-3′ orientation of the sequences shown in Table 2. The regions amplified by FIP and BIP primers of the N, Y5 and Y4 sets corresponded to nt 8808-8965, nt 8938-9217 and nt 8865-9027, respectively. Asterisks indicate the four nucleotide signatures specific to the O and N coat protein types, at nt 8890 and nt 8891 (GR for O type and AR for N type), nt 8905 (G for O type and A for N type), nt 8911 and nt 8912 (CU for O type and AC for N type) and nt 9035 (C for O type and A for N type). R represents either A or G
Strain identification by multiplex RT-PCR
Total RNA was isolated according to Zacharzewska et al. [16] from tobacco leaves (cv. Samsun) infected with PVY isolates Bonin 1-3, PVYO (PV-0345), PVYN-Wi (EF558545), PVYN (PV-0348), and PVYNTN (PV-0403). Reverse transcription reactions were carried out using a Reverse Super Verte KIT with random primers (Novazym Poland), following the manufacturer’s protocol. The resulting cDNA was next amplified by multiplex PCR according to Chikh-Ali et al. [4] with some modifications. The PCR mixture (10 µl) contained: 1-3 µl of cDNA; 0.2 U of uracil DNA glycosylase (UNG) (Bioline); 0.4 mM dNTPs (including dU instead of dT); 2 mM MgCl2; 0.2 mM primers n156, o514, n787, o2172, o2700; 0.1 mM primers n2258, n2650c; 0.6 mM primers n7577, SeroN, Y03-8648; 1 µl of 10-fold-concentrated polymerase buffer, and 0.6 U of GoTaq HotStart Polymerase (Promega). The temperature profile followed was as follows: UNG clean up at 40 °C for 2 min and initial denaturation at 94 °C for 1 min; 10 cycles at 94 °C for 30 s, 64 °C for 30 s, and 72 °C for 30 s; 10 cycles at 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s; 10 cycles at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by the final extension at 72 °C for 5 min. The reaction products were separated on a 2% agarose gel.
Real-time reverse transcription PCR
Real-time RT-PCR was conducted according to the two-step protocol developed by Singh et al. [13]. Total RNA was isolated by short magnetic capture and was reverse transcribed as described above. The real-time PCR was carried out on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Ltd.) using an AmpliQ Real-Time PCR OptiProbe kit or an AmpiQ Real-Time PCR SYBR Green kit (Novazym Polska s. c.). The final reactions (10 µl) contained 2.5 µl of cDNA, 5 µl of AmpiQ Real-Time PCR reagent, 0.15 µl of a 20 µM stock of forward and reverse primers, and 0.15 µl of 10 µM probe in the probe-based assays, or nuclease-free water in the dye-based assays. Different sets of primers were tested, including (i) the PVY-specific primers and TaqMan probe (TM) designed by Singh et al. [13], (ii) the Y4 F3 and B3 primers from the Y4 primer set (TM), combined with the TaqMan probe [13], and (iii) the Y4 F3 and B3 primers from the Y4 primer set in the presence of SYBR Green (SG) (for sequences, see Table 1). The conventional RT-PCR was performed with the Y4 F3 and B3 primers from the Y4 primer set. The thermal cycling steps were 10 min at 95 °C followed by 60 cycles of 10 s at 95 °C and 20 s at 60 °C. Reactions containing only water (no-template control) or cDNA transcribed from total RNA purified from virus-free plants were included in each run as controls. The fluorescence was monitored on the FAM channel and the results were interpreted in terms of Ct (cycle threshold values) determined used default settings in CFX Manager Software (Bio-Rad Ltd.).
Sensitivity of the RT-LAMP assay
The sensitivity of the RT-LAMP assay was determined by two separate approaches. The first approach was based on the isolation of total RNA by silica capture. The total RNA concentration was adjusted to 100 ng/ml, and tenfold dilutions were prepared down to 1 fg/ml. One microliter of the RNA sample was then amplified by RT-LAMP using the Y4, Y5 and N primer sets. The second approach was based on the serial dilution of the sap extracted from PVY-infected and healthy potato plants. We investigated (i) the impact of the isolation method on the sensitivity of the detection of PVY by the Y4-based RT-LAMP and (ii) the sensitivity of the Y4-based RT-LAMP when compared to real-time RT-PCR. The sap dilutions ranged from tenfold (dilution factor = 1 × 101) to two-million-fold (dilution factor = 2 × 106). For comparison of the sensitivity of the tested methods, the RNA isolation techniques, or the amplification procedures, total RNA was isolated from all prepared sap dilutions by magnetic capture. These RNAs were subsequently tested by RT-LAMP alone or also by real-time RT-PCR according to the procedures described above.
Comparison of detection methods using field samples
Thirty tubers from 30 offspring plants were harvested after planting secondarily PVY-infected and healthy tubers. The tubers were tested by the Y4-based RT-LAMP method as well as by probe-based and dye-based real-time PCR as described above using the magnetic III protocol for RNA extraction. For the standard grow-out (GO) tests, eye cores were cut, sprouted and grown in a glasshouse to produce 4- to 6 week-old offspring plants. Leaves from these plants were then tested for PVY using the double sandwich enzyme-linked immuno-absorbent assay (DAS-ELISA) according to the manufacturer’s protocol (Neogen).
Statistical analysis
Each experiment was conducted with at least two replicates and was repeated independently at least three times. A non-template control (sterile water), a negative control (total RNA extracted from a virus-free plant), and a positive control (total RNA extracted from a PVY-infected plant) were included for each LAMP/qPCR run. The time-to-positive (Tp) or Ct values were calculated using the manual baseline settings in Genie II (OptiGene Ltd) or CFX Manager Software (Bio-Rad Laboratories). Mean values and standard deviations were calculated, and the statistical analysis was carried out using GraphPad Prism 6.04 for Windows (GraphPad Software, La Jolla, California, USA, http://www.graphpad.com). An unpaired t-test was used to compare the mean annealing temperature (Ta) values recorded using a Genie II amplification system (OptiGene Ltd) or melting temperature (Tm) using a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Ltd) for PVY isolates representing the N or O coat protein type. Differences with p < 0.05 were considered statistically significant.
Results and discussion
Performance of the RT-LAMP primer sets
The high specificity of the RT-LAMP method relies heavily on the primer sets designed for the amplification of the targeted region. We thus performed a direct comparison of three RT-LAMP primer sets for PVY detection. These included two primer sets (N and Y5) previously reported in the literature [10, 11] and the newly designed Y4 set (Table 2 and Fig. 1), all of which targeted the coat protein region. While all primer sets successfully amplified the PVY targeted region as expected, their reaction rates were significantly different (Fig. 2a). The slowest amplification was observed for the N set, with a time-to-positive (Tp) value of 23 min. The Y5 primers had a Tp value equal to 17 min. The Y4 set gave the fastest PVY detection with a Tp value of 8 min (Fig. 2a). The specificity of all primer sets was confirmed by the lack of amplification from total RNA isolated from a mock-inoculated plant or a no-template control (Fig. 2a).
Performance of the Y4-set-primed RT-LAMP for PVY detection. (a) Comparison of the amplification speeds of the Y4, Y5 and N primer sets in the RT LAMP assay for PVY detection from total RNA isolated from plants infected with PVYNTN (isolate 12/94). We used total RNA from virus-free plants (nc) and a no-template control (ntc) as negative controls. The time-to-positive values are shown above the plot. (b) Comparison of the sensitivity of different RT-LAMP assays by serial dilution of total RNA. The RT-LAMP results are shown as time-to-positive (Tp) values as a function of the logarithm of the total RNA concentration. The total RNA (100 pg/µl) was serially diluted down to 0.001 pg/µl. The amounts of RNA [pg] detected by the specific RT-LAMP assays are indicated above the plot. (c) Specificity of the Y4 RT-LAMP for PVY detection expressed as amplification plots for reactions performed using total RNA isolated from plants infected with PVYNTN, PVA, PVM, PVS, PVX, or PLRV. (d) Second derivatives of annealing curves recorded for amplicons in the reactions described in panel c. The peak of the plot indicates the annealing temperature (Ta) of the amplicon produced by Y4 primers for a reaction containing PVYNTN RNA. The data are averages from at least two independent experiments performed in triplicate. Error bars indicate standard deviation
To compare the detection sensitivity achieved with the different primer sets, we performed a serial dilution of the total RNA, with the final amount of RNA ranging between 100 pg to 1 fg per reaction. We plotted the time-to-positive values for each primer set as a function of the logarithm of the RNA concentration (Fig. 2b), which showed a linear relationship for all primer sets. The highest sensitivity was obtained with the Y4 set, which successfully detected PVY with as little as 0.01 pg of RNA. Lower sensitivities by one (0.1 pg) and ten orders of magnitude (1 pg) were recorded for the Y5 and N sets, respectively.
The specificity of the Y4 primer set against PVY was confirmed by testing total RNA isolated from leaves infected with other viruses commonly infecting potato, including potato virus A (PVA), potato virus M (PVM), potato virus S (PVS), and potato virus X (PVX), along with PVY. As expected, only PVY was detected (Fig. 2c). No peak was detectable for any other viruses. The annealing temperature of the amplified RT-LAMP product was 84.4 °C (Figure 2d).
We concluded that the newly developed Y4 primer set specifically amplified the targeted region of PVY and that it yielded higher sensitivity than the other tested primer sets, including the previously described Y5 set, which had already been reported to be tenfold more sensitive than the RT-PCR and 1000-fold more sensitive than the enzyme immunoassays [11].
Discrimination of the PVY O and N types with the Y4 primer set
The ability to discriminate PVY isolates by strain or at least by serotype is a useful tool in disease diagnosis. We thus tested whether the Y4-based RT-LAMP assay could differentiate the PVY isolates that were positive in the multiplex RT-PCR (Fig. 3). These included different PVYO, PVYN-Wi, PVYN and PVYNTN isolates. We performed the RT-LAMP assay using a Genie II rapid amplification system and the Y4 and the Y5 primer sets (Fig. 4). While no amplification was observed when using total RNA isolated from virus-free plants, strong amplification was detected when using total RNA from all of the PVY-infected plants using both primer sets (Fig. 4a and b). We next assessed strain specificity by comparing the Ta values of each of the amplified products (Fig. 4c and e). The resulting curves revealed that the products amplified from PVY isolates with an O serotype, which included PVYN-Wi and PVYO, had approximately a 0.46 °C variation in their Ta values when compared to the amplified products from the N type isolates, which included PVYN and PVYNTN, but this was only observable with the Y4 primer set (p < 0.0001, Table 4). The average Ta value was about 84.37 °C for the N-type and 84.83 °C for the O-type (Table 3, Fig. 4c). Such significant temperature variation was not observed with the Y5 primer set (difference, 0.14 °C, p = 0.2191, Table 3, Fig. 4d and f). The average Ta value for all amplified products was 86.63 °C. Thus, the Y5 set detected PVY, but in contrast to the Y4 set, it failed to differentiate the PVY isolates (Table 3, Fig. 4d and f).
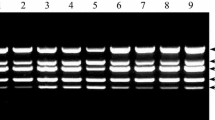
Identification of different PVY strains by multiplex RT-PCR. Lane 1, no-template control, lane 2, RNA from a healthy plant; lane 4, PV-0345; lane 5, Bonin 1; lane 6, Wi (EF558545); lanes 7 and 8, Bonin 2; lane 10, PV-0348 (X97895); lane 11, PV-0403; lanes 12 and 13, Bonin 3; lanes 3 and 9, molecular size markers (Nova 100 bp, Novazym Poland). The isolates PV-0345 and Bonin 1 were identified as PVYO, isolates Wi and Bonin 2 as PVYN-Wi, isolate PV-0348 as PVYN, isolates PV-0403 and Bonin 3 as PVYNTN
Differentiation of PVY types N and O by the Y4-primer-set-based RT-LAMP. Amplification plots recorded for RT-LAMP reactions with the Y4 (a) and Y5 (b) primer sets and total RNA isolated from plants infected with the indicated European PVY isolates, with total RNA from a virus-free plant (nc), or with water instead of RNA (ntc). Second derivatives of annealing curves recorded for amplicons amplified using the Y4 (c) and Y5 (d) primer sets in reactions described in panels a and b, respectively. Annealing temperature (Ta) ranges of the RT-LAMP products obtained on European PVY strains by amplification with the Y4 (e) and Y5 (f) primer sets. The annealing temperatures were recorded using a Genie II apparatus (g and h). Melting temperature (Tm) ranges of the RT-LAMP products obtained using European (g) or North American (h) PVY strains by amplification with the Y4 primer set. The melting temperatures were measured using the Bio-Rad apparatus. PVY strains with coat protein O or N types are indicated by symbols with a black or white background, respectively. The data are averages from at least two independent experiments performed in triplicate. Error bars indicate standard deviation
To determine whether the observed difference in Ta values with the Y4 primers (Fig. 4c and e) correlated with a difference in melting temperature (Tm), all PVY strains were tested using a real-time thermal cycler (Fig. 4g). Our data revealed that the PVY strains with an N-type coat protein sequence had a Tm value of 83.65 °C, while strains with an O-type coat protein sequence had a Tm value of 84.21 °C (difference, 0.56 °C, p < 0.0001, Table 3, Fig. 4g).
We expanded the RT-LAMP assays with the Y4 primers to include North American PVYO, PVYNN:O, PVYN-NA and PVYNTN isolates (Fig. 4h). The melting curves revealed that the products of the RT-LAMP amplification obtained for PVYO and PVYNN:O with the O-type coat protein gene had a Ta value (85.16 °C) that was 0.47 °C higher than that of the products obtained for PVYN-NA and PVYNTN (84.69 °C) (p = 0.0005, Table 3, Fig. 4h).
In summary, the Y4-based RT-LAMP set allowed us to discriminate PVY O and PVY N types in one reaction. Sequence analysis (Fig. 1) showed that the amplified region within the coat protein gene contains two single and two double nucleotide signatures typical for O and N coat protein types, which may be sufficient to contribute in the differences in annealing/melting temperatures that we observed between the N and O types (Table 3, Fig. 4).
Optimization of the RNA isolation procedure
Our next step was to streamline the RT-LAMP procedure. We thus compared different RNA isolation procedures, including magnetic capture and RNA silica capture, that we previously optimized for RT-PCR [13]. We first compared three versions of the magnetic-capture-based RNA isolation procedure as described in Materials and methods. These included (i) the magnetic capture following the manufacturer’s procedure, (ii) a modified magnetic capture with buffers adapted for silica capture [13], and (iii) a shortened modified magnetic capture with fewer washes and incubation steps than the modified capture procedure. We observed that all these procedures resulted in nearly identical amplification profiles in RT-LAMP (Fig. 5a). However, we shortened the procedure from start to completion from 30 and 60 min with the original and modified method, respectively, to 20 min with the shortened isolation approach.
Effect of the method of RNA isolation on the performance (a) and sensitivity (b) of detection of PVY by Y4 RT-LAMP. (a) Amplification plots recorded for RNA purified from plants infected with PVY by magnetic capture (i) according to the manufacturer (ii) by a modified magnetic procedure and (iii) by a shortened magnetic procedure. (b) Comparison of the effect of the different RNA isolation methods on sensitivity. Sap from plants infected with PVY was serially diluted up to 2 × 106 times, and total RNA was purified from each dilution by the magnetic capture procedure recommended by the manufacturer, by the shortened magnetic capture procedure, and by silica capture. Time-to-positive results are shown as a function of the logarithm of the dilution factor. The data are averages from at least two independent experiments performed in triplicate. Error bars indicate standard deviation
To test whether the technique of RNA isolation would influence the sensitivity of PVY detection by RT-LAMP, we compared the original magnetic capture method and the shorter magnetic capture method with the silica capture method, which resulted in high sensitivity in the RT-PCR assay [13] (Fig. 5b). Our data showed that all isolation methods had the same detection limit (a 1 × 105-fold dilution of sap), but the time-to-positive values were slightly higher with RNA isolated by magnetic capture than with RNA isolated by the silica-based RNA capture procedure (Fig. 5b).
In summary, we reduced the time required for the magnetic capture procedure for PVY detection by RT-LAMP from RNA isolation to assay to less than one hour without compromising the detection sensitivity.
Comparison of the sensitivity of PVY detection by RT-LAMP and by conventional and real-time RT-PCR
Next, we compared the sensitivity of the Y4-based RT-LAMP for PVY detection with that of real-time RT-PCR and conventional RT-PCR using serial dilutions of sap. The data revealed that the RT-LAMP was as sensitive as all investigated variants of the real-time RT-PCR, with the detection of PVY in sap diluted up to 2 × 106 fold in fewer than 40 cycles (Fig. 6a). However, the detection of PVY at the highest RNA concentration (1 × 102-fold dilution) required more than 25 cycles for all tested real-time RT-PCR variants but only 19 cycles by RT-LAMP. To contrast, the conventional RT-PCR amplified a PCR product of the expected size in samples diluted only up to 1 × 106-fold (Fig. 6b).
Comparison of sensitivity of the Y4 RT-LAMP, the TaqMan real-time RT-PCR, the Y4 TaqMan real-time RT-PCR, the Y4 SYBR Green real-time PCR (a), and the conventional Y4 RT-PCR (b). (a) For the real-time tests, Cq results are shown as a function of the logarithm of the dilution factor. The data are averages from at least two independent experiments performed in triplicate, and error bars indicate standard deviation. (b) Determination of the sensitivity of PVY detection by RT-PCR in sap from a PVY-infected plant diluted 1 × 102, lane 3; 1 × 103, lane 4; 1 × 104, lane 5; 5 × 104, lane 6; 1 × 105, lane 7; 5 × 105, lane 8; 1 × 106, lane 9; 2 × 106, lane 10. No-template control (PCR reaction containing water instead of cDNA), lane 1; negative control (RT-PCR reaction containing RNA from a virus-free plant), lane 2; Nova 100-bp molecular weight marker (Novazym Poland), lanes M
While the RT-LAMP assay shares similar sensitivity with the standard real-time RT-PCR assay, it is clearly faster. The detection of PVY in the highest dilution of sap can be accomplished in 15-60 min by RT-LAMP with its 30-s cycles. In contrast, the completion of the real-time RT-PCR reaction with the same sample requires 2-3 h.
Reliability of PVY detection in dormant tubers
To estimate the reliability of the RT-LAMP for detection of PVY in dormant tubers, tubers collected from secondarily infected plants were tested for the presence of PVY by RT-LAMP (Y4), by the two variants of real-time RT-PCR (dye-based [SG] and probe-based [TM]) and by the standard grow-out test (GO). While all 30 tubers tested positive for PVY by the GO test, 26 tubers tested positive by RT-LAMP while only 19 tubers tested positive by both variants of real-time RT-PCR (Table 4). Our data suggest that while the grow-out test remains a reliable method for the detection of PVY in dormant tubers, the RT-LAMP assay clearly out-performed the real-time RT-PCR assay. This may be linked to the requirement for high-quality RNA preparations for PCR-based amplification, which can be a challenge with the low virus titer in dormant tubers [3]. It is worth noting that some differences were observed between the real-time PCR variants depending on whether the detection was dye- or probe-based (Table 4).
References
Almasi MA, Dehabadi SH (2013) Colorimetric immunocapture reverse transcription loop-mediated isothermal amplification assay for rapid detection of the Potato virus Y. Plant Pathol Microbiol. 4:188–193
Benedict CA, McMoran DW, Inglis DA, Karasev AV (2015) Tuber symptoms associated with recombinant strains of Potato virus Y in specialty potatoes under Western Washington growing conditions. Am J Potato Res 92:593–602
Boonham N, Glover R, Tomlinson J, Mumford R (2008) Exploiting generic platform technologies for the detection and identification of plant pathogens. Eur J Plant Pathol 121:355–363
Chikh Ali M, Maoka T, Natsuaki KT, Natsuaki T (2010) The simultaneous differentiation of Potato virus Y strains including the newly described strain PVY(NTN-NW) by multiplex PCR assay. J Virol Methods 165:15–20
Funke CN, Nikolaeva OV, Green KJ, Tran LT, Chikh-Ali M, Quintero-Ferrer A, Cating RA, Frost KE, Hamm PB, Olsen N, Pavek MJ, Gray SM, Crosslin JM, Karasev AV (2017) Strain-specific resistance to Potato virus Y (PVY) in potato and its effect on the relative abundance of PVY strains in commercial potato fields. Plant Dis 101:20–28
Hühnlein A, Drechsler N, Steinbach P, Thieme P, Schubert J (2013) Comparison of three methods for the detection of potato virus Y in seed potato certification. J Plant Dis Protect 120:57–69
Karasev AV, Hu X, Brown CJ, Kerlan C, Nikolaeva OV, Crosslin JM, Gray SM (2011) Genetic diversity of the ordinary strain of Potato virus Y (PVY) and origin of recombinant PVY strains. Phytopathology 101:778–785
Karasev AV, Gray SM (2013) Continuous and emerging challenges of Potato virus Y in potato. Ann Rev Phytopathol 51:571–586
Kerlan C, Moury B (2008) Potato virus Y. Encycl Virol 4:287–296
Nie X (2005) Reverse transcription loop-mediated isothermal amplification of DNA for detection of Potato virus Y. Plant Dis 89:605–610
Przewodowska A, Zacharzewska B, Chołuj J, Treder K (2015) A one-step, real-time reverse transcription loop-mediated isothermal amplification assay to detect Potato virus Y. Am J Potato Res 92:303–311
Scholthof KBG, Adkins S, Czosnek H, Palukaitis P, Jacquot E, Hohn T, Hohn B, Saunders K, Candresse T, Ahlquist P, Hemenway C, Foster GD (2011) Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol 12:938–954
Singh M, Singh RP, Fageria MS, Nie X, Coffin R, Hawkins G (2013) Optimization of a real-time RT PCR assay and its comparison with ELISA, conventional RT-PCR and the grow-out test for large scale diagnosis of potato virus Y in dormant potato tubers. Am J Potato Res 90:43–50
Singh RP (1999) Development of the molecular methods for potato virus and viroid detection and prevention. Genome 42:592–604
Treder K, Przewodowski W, Barnyk A (2009) Factors influencing detection of Potato leafroll virus and Potato virus Yin potato tuber extracts. Plant Breed Seed Sci 59:65–74
Zacharzewska B, Przewodowska A, Treder K (2014) The adaptation of silica capture RT-PCR for the detection of Potato virus Y. Am J Potato Res 91:525–531
Acknowledgements
The authors would like to thank Prof. Renata Lebecka and Prof. Ewa Zimnoch-Guzowska for providing tubers infected with PVY isolates, and MSc. Danuta Sekrecka for providing virus-free plants and sources of PLRV, PVA, PVM, PVS and PVX. We also want to thank Maria Fedczak for excellent technical help.
Author information
Authors and Affiliations
Contributions
KT conceived of and designed the study. KT, JCH, LB, BZ, MM, AB performed research. KT and AMR analyzed data. AMR and KT wrote the paper.
Corresponding authors
Ethics declarations
Funding
This study was supported financially by the Ministry of Agriculture and Rural Development, Poland, project no. 58: “Development of sensitive methods for detection of most important potato viruses” (4-3-00-7-01), and by the National Science Centre, Poland (Grant no. 2016/21/B/NZ9/03573) to KT, and by a Hatch Act Fund (MSN179998) and a Wisconsin Potato Industry Board Grant (MSN191752) to AMR.
Conflict of interest
AB is CEO of Novazym Polska s. c. The other authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participation or animals performed by any of the authors.
Additional information
Handling Editor: Ralf Georg Dietzgen.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Treder, K., Chołuj, J., Zacharzewska, B. et al. Optimization of a magnetic capture RT-LAMP assay for fast and real-time detection of potato virus Y and differentiation of N and O serotypes. Arch Virol 163, 447–458 (2018). https://doi.org/10.1007/s00705-017-3635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3635-3