Abstract
Human leukocyte antigen (HLA) alleles are associated with both the progression of chronic hepatitis C (CHC) and the sustained virological response (SVR) to antiviral therapy. HLA-A*02 is the most common HLA allele in people of European/Caucasian descent and the Chinese and Japanese population. Therefore, we investigated whether HLA-A*02 expression is associated with disease outcome in Chinese CHC patients. Three hundred thirty-one treatment-naïve CHC patients were recruited in this study. The expression of HLA-A*02 was tested by FACS and LABType SSO assays. All patients were treated weekly with pegylated interferon plus ribavirin (PEG-IFN/RBV) according to a standard protocol. Virological response was assessed by TaqMan assay at the 4th, 12th, 24th, and 48th week of therapy, and again at the 24th week post-therapy. By the end of the study, 293 CHC patients, including 144 HLA-A*02-positive patients and 149 HLA-A*02-negative patients, were evaluable for analysis. There were no statistical differences in clinicopathological parameters between HLA-A*02-positive and negative patients before antiviral therapy (P > 0.05). The HLA-A*02-positive patients had a higher rapid virological response (RVR, 74.3 % versus 62.4 %, P = 0.03) and SVR (78.5 % versus 64.4 %, P = 0.01) and a lower relapse rate (4.2 % versus 11.9 %, P = 0.03) than HLA-A*02-negative patients. Multivariable logistic regression analysis showed that HLA-A*02 expression, liver fibrosis stages <S3, HCV genotype 2a, IL-28B rs8099917 TT, and RVR were independent predictive factors of SVR (P < 0.05). Host HLA-A*02 allele expression is associated with SVR, highlighting the importance of considering HLA-A*02 as a predictor of the response to PEG-IFN/RBV treatment in the Chinese population with CHC.


Similar content being viewed by others
References
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57:1333–1342
Munir S, Saleem S, Idrees M, Tariq A, Butt S, Rauff B, Hussain A, Badar S, Naudhani M, Fatima Z, Ali M, Ali L, Akram M, Aftab M, Khubaib B, Awan Z (2010) Hepatitis C treatment: current and future perspectives. Virol J 7:296
Poynard T, Yuen MF, Ratziu V, Lai CL (2003) Viral hepatitis C. Lancet 362:2095–2100
Lawitz E, Gane EJ (2013) Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 369:678–679
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM (2014) Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 370:211–221
Younossi ZM, Kanwal F, Saab S, Brown KA, El-Serag HB, Kim WR, Ahmed A, Kugelmas M, Gordon SC (2014) The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 39:518–531
Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD (2014) Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. Incarcerated populations: a cost-effectiveness analysis. Ann Intern Med 161:546–553
Aman W, Mousa S, Shiha G, Mousa SA (2012) Current status and future directions in the management of chronic hepatitis C. Virol J 9:57
Shepard CW, Finelli L, Alter MJ (2005) Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5:558–567
Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ (2007) Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol 81:5882–5892
Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B (2000) Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med 6:578–582
Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV (2001) Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 194:1395–1406
Zubkova I, Duan H, Wells F, Mostowski H, Chang E, Pirollo K, Krawczynski K, Lanford R, Major M (2014) Hepatitis C virus clearance correlates with HLA-DR expression on proliferating CD8+ T cells in immune-primed chimpanzees. Hepatology 59:803–813
Wang JH, Zheng X, Ke X, Dorak MT, Shen J, Boodram B, O’Gorman M, Beaman K, Cotler SJ, Hershow R, Rong L (2009) Ethnic and geographical differences in HLA associations with the outcome of hepatitis C virus infection. Virol J 6:46
Neumann-Haefelin C, Frick DN, Wang JJ, Pybus OG, Salloum S, Narula GS, Eckart A, Biezynski A, Eiermann T, Klenerman P, Viazov S, Roggendorf M, Thimme R, Reiser M, Timm J (2008) Analysis of the evolutionary forces in an immunodominant CD8 epitope in hepatitis C virus at a population level. J Virol 82:3438–3451
Satapathy SK, Lingisetty CS, Proper S, Chaudhari S, Williams S (2010) Equally poor outcomes to pegylated interferon-based therapy in African Americans and Hispanics with chronic hepatitis C infection. J Clin Gastroenterol 44:140–145
Kobayashi K, Ishii M, Shiina M, Ueno Y, Kondo Y, Kanno A, Miyazaki Y, Yamamoto T, Kobayashi T, Niitsuma H, Kikumoto Y, Takizawa H, Shimosegawa T (2005) Interferon-gamma is produced by CD8 T cells in response to HLA-A24-restricted hepatitis C virus epitopes after sustained virus loss. Clin Exp Immunol 141:81–88
Singh R, Kaul R, Kaul A, Khan K (2007) A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J Gastroenterol 13:1770–1787
McKiernan SM, Hagan R, Curry M, McDonald GS, Kelly A, Nolan N, Walsh A, Hegarty J, Lawlor E, Kelleher D (2004) Distinct MHC class I and II alleles are associated with hepatitis C viral clearance, originating from a single source. Hepatology 40:108–114
Harris RA, Sugimoto K, Kaplan DE, Ikeda F, Kamoun M, Chang KM (2008) Human leukocyte antigen class II associations with hepatitis C virus clearance and virus-specific CD4 T cell response among Caucasians and African Americans. Hepatology 48:70–79
Thio CL, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O’Brien SJ, Karacki P, Marti D, Astemborski J, Carrington M (2001) Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis 184:16–21
Middleton D, Menchaca L, Rood H, Komerofsky R (2003) New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens 61:403–407
Cheng LH, Jin SZ, Gao SQ, Li Z, Zou HY, Wang DM, Wu GG (2005) Difference in HLA-A*02 allele distribution between Han populations in south and north China. Di Yi Jun Yi Da Xue Xue Bao 25:321–324
Kondo Y, Kobayashi K, Kobayashi T, Shiina M, Ueno Y, Satoh T, Shimosegawa T (2003) Distribution of the HLA class I allele in chronic hepatitis C and its association with serum ALT level in chronic hepatitis C. Tohoku J Exp Med 201:109–117
(2011) EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 55:245–264
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293
Umemura T, Joshita S, Yoneda S, Katsuyama Y, Ichijo T, Matsumoto A, Yoshizawa K, Ota M, Tanaka E (2011) Serum interleukin (IL)-10 and IL-12 levels and IL28B gene polymorphisms: pretreatment prediction of treatment failure in chronic hepatitis C. Antivir Ther 16:1073–1080
Dai CY, Chuang WL, Hsieh MY, Huang JF, Lin YY, Chu PY, Hou NJ, Lin ZY, Chen SC, Wang LY, Yu ML (2010) Human leukocyte antigen alleles and the response to pegylated interferon/ribavirin therapy in chronic hepatitis C patients. Antiviral Res 85:396–402
McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS (2009) Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 361:580–593
Boghdadi G, Seleem WM (2014) Differential expression of Toll-like receptors 7 & 8 mRNA in monocytes of patients with chronic hepatitis C infection: correlation with interferon and ribavirin treatment. Egypt J Immunol 21:67–75
Imran M, Manzoor S, Parvaiz F (2014) Predictive potential of IL-18 -607 and osteopontin −442 polymorphism in interferon-based therapy of HCV infection in the Pakistani population. Viral Immunol 27:404–411
Eslam M, Leung R, Romero-Gomez M, Mangia A, Irving WL, Sheridan D, Spengler U, Mollison L, Cheng W, Bugianesi E, McLeod D, Zaitoun AM, Attino V, Goeltz D, Nattermann J, Douglas M, Booth DR, George J, Ahlenstiel G (2014) IFNL3 polymorphisms predict response to therapy in chronic hepatitis C genotype 2/3 infection. J Hepatol 61:235–241
Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O’Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DM, Vine S, Addo MM, Allen TM, Altfeld M, Henn MR, Le Gall S, Streeck H, Haas DW, Kuritzkes DR, Robbins GK, Shafer RW, Gulick RM, Shikuma CM, Haubrich R, Riddler S, Sax PE, Daar ES, Ribaudo HJ, Agan B, Agarwal S, Ahern RL, Allen BL, Altidor S, Altschuler EL, Ambardar S, Anastos K, Anderson B, Anderson V, Andrady U, Antoniskis D, Bangsberg D, Barbaro D, Barrie W, Bartczak J, Barton S, Basden P, Basgoz N, Bazner S, Bellos NC, Benson AM, Berger J, Bernard NF, Bernard AM, Birch C, Bodner SJ, Bolan RK, Boudreaux ET, Bradley M, Braun JF, Brndjar JE, Brown SJ, Brown K, Brown ST, Burack J, Bush LM, Cafaro V, Campbell O, Campbell J, Carlson RH, Carmichael JK, Casey KK, Cavacuiti C, Celestin G, Chambers ST, Chez N, Chirch LM, Cimoch PJ, Cohen D, Cohn LE, Conway B, Cooper DA, Cornelson B, Cox DT, Cristofano MV, Cuchural G, Jr., Czartoski JL, Dahman JM, Daly JS, Davis BT, Davis K, Davod SM, DeJesus E, Dietz CA, Dunham E, Dunn ME, Ellerin TB, Eron JJ, Fangman JJ, Farel CE, Ferlazzo H, Fidler S, Fleenor-Ford A, Frankel R, Freedberg KA, French NK, Fuchs JD, Fuller JD, Gaberman J, Gallant JE, Gandhi RT, Garcia E, Garmon D, Gathe JC, Jr., Gaultier CR, Gebre W, Gilman FD, Gilson I, Goepfert PA, Gottlieb MS, Goulston C, Groger RK, Gurley TD, Haber S, Hardwicke R, Hardy WD, Harrigan PR, Hawkins TN, Heath S, Hecht FM, Henry WK, Hladek M, Hoffman RP, Horton JM, Hsu RK, Huhn GD, Hunt P, Hupert MJ, Illeman ML, Jaeger H, Jellinger RM, John M, Johnson JA, Johnson KL, Johnson H, Johnson K, Joly J, Jordan WC, Kauffman CA, Khanlou H, Killian RK, Kim AY, Kim DD, Kinder CA, Kirchner JT, Kogelman L, Kojic EM, Korthuis PT, Kurisu W, Kwon DS, LaMar M, Lampiris H, Lanzafame M, Lederman MM, Lee DM, Lee JM, Lee MJ, Lee ET, Lemoine J, Levy JA, Llibre JM, Liguori MA, Little SJ, Liu AY, Lopez AJ, Loutfy MR, Loy D, Mohammed DY, Man A, Mansour MK, Marconi VC, Markowitz M, Marques R, Martin JN, Martin HL, Jr., Mayer KH, McElrath MJ, McGhee TA, McGovern BH, McGowan K, McIntyre D, McLeod GX, Menezes P, Mesa G, Metroka CE, Meyer-Olson D, Miller AO, Montgomery K, Mounzer KC, Nagami EH, Nagin I, Nahass RG, Nelson MO, Nielsen C, Norene DL, O’Connor DH, Ojikutu BO, Okulicz J, Oladehin OO, Oldfield EC, 3rd, Olender SA, Ostrowski M, Owen WF, Jr., Pae E, Parsonnet J, Pavlatos AM, Perlmutter AM, Pierce MN, Pincus JM, Pisani L, Price LJ, Proia L, Prokesch RC, Pujet HC, Ramgopal M, Rathod A, Rausch M, Ravishankar J, Rhame FS, Richards CS, Richman DD, Rodes B, Rodriguez M, Rose RC, 3rd, Rosenberg ES, Rosenthal D, Ross PE, Rubin DS, Rumbaugh E, Saenz L, Salvaggio MR, Sanchez WC, Sanjana VM, Santiago S, Schmidt W, Schuitemaker H, Sestak PM, Shalit P, Shay W, Shirvani VN, Silebi VI, Sizemore JM, Jr., Skolnik PR, Sokol-Anderson M, Sosman JM, Stabile P, Stapleton JT, Starrett S, Stein F, Stellbrink HJ, Sterman FL, Stone VE, Stone DR, Tambussi G, Taplitz RA, Tedaldi EM, Theisen W, Torres R, Tosiello L, Tremblay C, Tribble MA, Trinh PD, Tsao A, Ueda P, Vaccaro A, Valadas E, Vanig TJ, Vecino I, Vega VM, Veikley W, Wade BH, Walworth C, Wanidworanun C, Ward DJ, Warner DA, Weber RD, Webster D, Weis S, Wheeler DA, White DJ, Wilkins E, Winston A, Wlodaver CG, van’t Wout A, Wright DP, Yang OO, Yurdin DL, Zabukovic BW, Zachary KC, Zeeman B, Zhao M (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557
Song H, Pavlicek JW, Cai F, Bhattacharya T, Li H, Iyer SS, Bar KJ, Decker JM, Goonetilleke N, Liu MK, Berg A, Hora B, Drinker MS, Eudailey J, Pickeral J, Moody MA, Ferrari G, McMichael A, Perelson AS, Shaw GM, Hahn BH, Haynes BF, Gao F (2012) Impact of immune escape mutations on HIV-1 fitness in the context of the cognate transmitted/founder genome. Retrovirology 9:89
Shea PR, Shianna KV, Carrington M, Goldstein DB (2013) Host genetics of HIV acquisition and viral control. Annu Rev Med 64:203–217
Macnamara A, Rowan A, Hilburn S, Kadolsky U, Fujiwara H, Suemori K, Yasukawa M, Taylor G, Bangham CR, Asquith B (2010) HLA class I binding of HBZ determines outcome in HTLV-1 infection. PLoS Pathog 6:e1001117
Khakoo SI, Carrington M (2006) KIR and disease: a model system or system of models? Immunol Rev 214:186–201
Goodridge JP, Lee N, Burian A, Pyo CW, Tykodi SS, Warren EH, Yee C, Riddell SR, Geraghty DE (2013) HLA-F and MHC-I open conformers cooperate in a MHC-I antigen cross-presentation pathway. J Immunol 191:1567–1577
Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Poland GA (2012) Consistency of HLA associations between two independent measles vaccine cohorts: a replication study. Vaccine 30:2146–2152
Poland GA, Ovsyannikova IG, Jacobson RM (2008) Immunogenetics of seasonal influenza vaccine response. Vaccine 26(Suppl 4):D35–D40
Lancaster T, Sanders E, Christie JM, Brooks C, Green S, Rosenberg WM (2002) Quantitative and functional differences in CD8+ lymphocyte responses in resolved acute and chronic hepatitis C virus infection. J Viral Hepat 9:18–28
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M (2009) Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 41:1105–1109
Pellicelli AM, Romano M, Stroffolini T, Mazzoni E, Mecenate F, Monarca R, Picardi A, Bonaventura ME, Mastropietro C, Vignally P, Andreoli A, Marignani M, D’Ambrosio C, Miglioresi L, Nosotti L, Mitidieri O, Gentilucci UV, Puoti C, Barbaro G, Barlattani A, Furlan C, Barbarini G (2012) HCV genotype 1a shows a better virological response to antiviral therapy than HCV genotype 1b. BMC Gastroenterol 12:162
Kim IH (2010) Peginterferon alfa-2a/ribavirin for 48 or 72 weeks in hepatitis C genotype 1 and 4 patients with slow virologic response. Korean J Hepatol 16:201–205
Ma P, Yang JM, Hou W, Song SD, Wang L, Lu W (2013) A preliminary study on the efficacy and influencing factors of interferon for the treatment of genotype 1 chronic hepatitis C with different dosage forms. Eur J Gastroenterol Hepatol 25:601–605
Zhou JY, Jiang ZA, Zhou DF, Xing WL, Wang W, Wang YD (2013) Virological responses in chronic hepatitis C patients undergoing prolonged therapy with low-dose Peg-IFNalpha-2a. Hepatogastroenterology 60:301–304
Sarwar S, Ryan EJ, Iqbal M, McCormick PA, O’Farrelly C, Hegarty J (2012) Rapid, early and sustained virological responses in a cohort of Irish patients treated with pegylated interferon and ribavirin for chronic hepatitis C virus infection. Ir J Med Sci 181:53–58
Peng CY, Chen TH, Lim YP, Tsai FJ, Lin WY, Liao WL, Wan L (2014) Association of MRC-1 and IL-28B with the treatment outcome of hepatitis C: a case control study. BMC Gastroenterol 14:113
Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J (2009) IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 41:1100–1104
Mangia A, Mottola L, Santoro R (2013) Interleukin 28B polymorphisms as predictor of response in hepatitis C virus genotype 2 and 3 infected patients. World J Gastroenterol 19:8924–8928
Sarrazin C, Susser S, Doehring A, Lange CM, Muller T, Schlecker C, Herrmann E, Lotsch J, Berg T (2011) Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients. J Hepatol 54:415–421
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M (2009) Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461:798–801
Chen H, Zhang Y, Huang P, Xu Y, Wang J, Su J, Yu R (2014) Host genetic variations are associated with virological response to interferon therapy of chronic HCV in Han Chinese patients. J Biomed Res 28:476–483
Mi Y, Gao YT, Jiao XL, Guo H, Liu T, Jing L, Shi WX, Du Z (2014) The role of interleukin-28b gene polymorphisms in chinese patients with chronic hepatitis C treated with pegylated interferon and ribavirin. Hepat Mon 14:e18793
Dong ZX, Zhou HJ, Xiang XG, Guo SM, Zhuang Y, Zhao GD, Xie Q (2014) IL28B genetic variations are associated with treatment response of patients with chronic hepatitis C in Chinese Han population. J Dig Dis 14(10):1111
Liu T, Sha K, Yang L, Wang Y, Zhang L, Liu X, Yang F (2014) IL-28B polymorphisms correlated with treatment response in HCV-4 mono-infected patients: a meta-analysis. PLoS One 9:e91316
Cheng WS, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, Rawlinson W, Marks PS, Thommes J, Rizkalla B, Yoshihara M, Dore GJ (2010) Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol 53:616–623
Gordon SC, Muir AJ, Lim JK, Pearlman B, Argo CK, Ramani A, Maliakkal B, Alam I, Stewart TG, Vainorius M, Peter J, Nelson DR, Fried MW, Rajender Reddy K (2014) Safety profile of boceprevir and telaprevir in chronic hepatitis C: real-world experience from HCV-TARGET. J Hepatol 10:168–180
Ferreira PR, da Silva MH, Brandao-Melo CE, Rezende RE, Gonzalez M, Reuter T, Urbaez JD, Gianini RJ, Martinelli A, Mendes-Correa MC (2014) The clinical effectiveness of pegylated interferon and ribavirin for the treatment of chronic hepatitis C in HIV-infected patients in Brazil: a multicentric study. Br J Infect Dis 14:166–174
Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, Sankoh AJ, Adda N, Kauffman RS, George S, Wright CI, Poordad F (2011) Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 365:1014–1024
Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364:1207–1217
Pawlotsky JM (2011) The results of Phase III clinical trials with telaprevir and boceprevir presented at the Liver Meeting 2010: a new standard of care for hepatitis C virus genotype 1 infection, but with issues still pending. Gastroenterology 140:746–754
Reau NS, Jensen DM (2014) Sticker shock and the price of new therapies for hepatitis C: is it worth it? Hepatology 59:1246–1249
Mao Wenhui, Chen Wen, Wei Lai (2012) Cost-effectiveness analysis of Peg-interferon Alpha-2a plus ribavirin vs. conventional interferon plus ribavirin for the treatment of chronic hepatitis C in China. China J Pharm Econ 15:6–14
Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R (2013) Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 57:2164–2170
Yu ML, Chuang WL (2009) Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol 24:336–345
Huang CF, Huang JF, Yang JF, Hsieh MY, Lin ZY, Chen SC, Wang LY, Juo SH, Chen KC, Chuang WL, Kuo HT, Dai CY, Yu ML (2012) Interleukin-28B genetic variants in identification of hepatitis C virus genotype 1 patients responding to 24 weeks peginterferon/ribavirin. J Hepatol 56:34–40
Acknowledgments
We thank all the clinical staff in the Biotherapy Center for their assistance in experiments. In addition, we thank the staff in the Department of Infectious Disease and the Department of Gastroenterology for their assiduous collection of blood samples and data of patients. Finally, we are grateful to all of the participants and their families for generously agreeing to take part in this study. The work was supported by grants from the National Natural Science Foundation of China (Grant no. 81271815), Research Grant from the Ministry of Public Health (Grant no. 20110110001), the Basic and Advanced Technology Research Foundation from Science and Technology Department of Henan Province (Grant nos. 112300410153 and 122300410155), Funds for Creative Research Team of Henan Province, Creative Research Team of Higher Education of Henan Province and the Innovation Team of the First Affiliated Hospital of Zhengzhou University, Henan, China.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2015_2361_MOESM1_ESM.tif
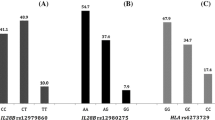
HLA-A*02 expression in CHC patients. (A) The HLA-A*02 of CD3+ CD8+ T cells was tested by FACS Cano II. (B) The HLA-A*02 expression in five CHC patients was tested by SSO LABType. The products were electrophoresed in a 1 % agarose gel and visualized by ethidium bromide staining. The outer pair of primers produced a 511-bp PCR product, and the inner pair of primers produced a 236-bp PCR product (TIFF 296 kb)
Rights and permissions
About this article
Cite this article
Wang, M., Li, Js., Ping, Y. et al. The host HLA-A*02 allele is associated with the response to pegylated interferon and ribavirin in patients with chronic hepatitis C virus infection. Arch Virol 160, 1043–1054 (2015). https://doi.org/10.1007/s00705-015-2361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2361-y