Abstract
Porcine parvovirus (PPV) causes reproductive failure in pigs, which leads to economic losses to the industry. As reported previously, LiCl efficiently impairs the replication of a variety of viruses, including the coronavirus infectious bronchitis virus (IBV), transmissible gastroenteritis virus (TGEV), and pseudorabies herpesvirus. We demonstrate for the first time that inhibition of PPV replication in swine testis (ST) cells by LiCl is dose-dependent, and that the antiviral effect of LiCl occurred in the early phase of PPV replication. These results indicate that LiCl might be an effective anti-PPV drug to control PPV disease. Further studies are required to explore the mechanism of the antiviral effect of LiCl on PPV infection in vivo.
Similar content being viewed by others
Introduction
Porcine parvovirus (PPV) is a member of the family Parvoviridae, subfamily Parvovirinae, genus Protoparvovirus. The parvoviruses are small, non-enveloped, single-stranded DNA viruses. PPV is a major cause of reproductive failure in sows and is a significant cofactor in porcine post-weaning multisystemic wasting syndrome [1–3]. PPV was first isolated from pigs with fever in 1965 [4]. It is commonly found in diseased pigs and is the cause of significant economic loss for the swine industry today [1, 5–9]. Currently, vaccination is the primary measure for prevention of PPV. However, the costs of vaccinations are high and might lead to biological safety issues, such as recombination and mutation of viruses. Additionally, even with vaccination, PPV is widespread in swine. Although vaccination can prevent the clinical symptoms of the PPV disease, it cannot eliminate the impairment caused by PPV disease [10, 11]. Therefore, alternative strategies, such as drug therapies to control the PPV, should receive increased attention.
Based on early research, tharide (LiCl) is used as a drug for treatment of bipolar disorder, depression and Alzheimer’s disease, and fracture healing has been observed in rodents treated with LiCl [12–15]. Recently, LiCl showed antiviral effects on cells infected with infectious bronchitis virus (IBV), an avian coronavirus, transmissible gastroenteritis virus (TGEV), and pseudorabies herpesvirus [16–18], which indicated that LiCl has potential as an antiviral drug. However, it is not known whether LiCl has an inhibitory effect on PPV. In this study, the antiviral activity of LiCl on PPV was investigated in vitro, and found to significantly inhibit PPV replication.
Materials and methods
The virus, the cells and the drug
PPV was isolated from a pig farm in Guangdong Province, China. In March of 2013, a PPV with low virulence, named PPV2013, was isolated from a pool of tissues (including lung, liver and kidney) of dead foetuses from a sow experiencing reproductive losses such as mummification, stillborns and weak foetuses. This sow came from a commercial pig farm located in Guangdong Province (Southern China). ST cells were obtained from the American Type Culture Collection (ATCC) and were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco, USA) containing 10 % fetal bovine serum (FBS) at 37 °C and 5 % CO2. LiCl was purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA) and was prepared in DMEM at a concentration of 1 M and sterilized by passage through a 0.22-µm filter.
Cytotoxicity assay
ST cells were cultured in 96-well plates (1 × 104 cells per well) and grown in Dulbecco’s modified Eagle medium (DMEM) with 10 % fetal bovine serum at 37 °C and 5 % CO2. Cytotoxicity assays were performed according to the instructions of the manufacturer for the Cell Counting Kit-8 (CCK8; Donjindo, Japan). Briefly, the ST cells in the 96-well culture plates were washed three times with PBS and were then incubated with 100 µl of LiCl in a series of concentrations (10, 20, 25, 30, 50, 80, and 100 mM) in serum-free EMEM (six wells/dilution) for 48–72 h. Mock-treated ST cells served as controls. After washing with PBS, the ST cells were incubated with medium (80 µl per well) and CCK8 solution (20 µl per well) at 37 °C for 1–4 h. The optical density (OD) was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad, USA). The relative cell viability rate was determined for each concentration as (OD450 drug)/(OD450 control) × 100. LiCl concentrations below the 50 % cytostatic concentration (CC50) were defined as non-toxic concentrations [19].
Assays for examining the effect of drug addition at different stages course of infection
Viral attachment stage
To evaluate whether LiCl affects attachment of PPV to ST cells, ST cells in 24-well plates were infected with PPV (MOI = 0.1) and treated with nontoxic concentrations (0, 5, 10, 15, 20, 25, and 30 mM) of LiCl for 1 h at 4 °C, a temperature that allowed viruses to bind to the surface of cells but not enter them. After removing unbound viruses by washing with PBS, the ST cell lysates were prepared by three cycles of freezing and thawing, and the viral loads were then calculated.
Viral entry stage
To determine whether LiCl affected entry of PPV into ST cells, ST cells in 24-well plates were infected with PPV (MOI = 0.1) at 4 °C for 1 h. After removing unbound virus by washing with PBS, the ST cells were treated with nontoxic concentrations (0, 5, 10, 15, 20, 25, and 30 mM) of LiCl at 37 °C for 1 h. Subsequently, the drug was removed, and the ST cells were grown in fresh medium for 24 h. The ST cell lysates were prepared by three cycles of freezing and thawing, and the viral loads were then calculated.
Viral replication stage
To determine whether LiCl affected the replication of PPV in ST cells, ST cells in 24-well plates were infected with PPV (MOI = 0.1) at 37 °C for 1 h to allow virus entry. After removing unbound virus by washing with PBS, ST cells were treated with nontoxic concentrations (0, 5, 10, 15, 20, 25, and 30 mM) of LiCl at 37 °C for 24 h. Subsequently, the ST cell lysates were prepared by three cycles of freezing and thawing, and the viral loads were then calculated.
Assay for examining the effect of drug addition at different times after infection
To evaluate at which time after infection viral replication was impacted by the drug, ST cells in 24-well plates were infected with PPV (MOI = 0.1) at 37 °C for 1 h. The ST cells were then washed three times with PBS, grown in fresh medium (set as 0 h), and LiCl was added to the cells at the nontoxic concentration of 30 mM of at 0, 1, 3, 6, 9, 12, and 18 hours postinfection, which included the most drug-sensitive phase of virus replication. ST cell lysates were prepared by three cycles of freezing and thawing, and viral loads were calculated at 24 h postinfection.
Virus titration
ST cells were seeded into 96-well plates 24 h before infection. Cell lysates from the experiments were serially diluted tenfold in DMEM and were added to the ST cells in five wells. After incubation at 37 °C for 72 h, the 50 % tissue culture infectious dose (TCID50) was calculated by the method of Reed and Muench.
Real-time PCR
To confirm the inhibitory effects of LiCl on PPV replication, the ST cell lysates from the viral replication stage assays were subjected to real-time PCR. Total DNA was extracted with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the instructions of the manufacturer. cDNA was made using a Thermo Scientific Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). Real-time quantitative PCR, targeting the NS1 gene of PPV was carried out with the primers shown in Table 1, using a 7500 Real Time PCR System (Applied Biosystems, USA) and a SYBR Select Master Mix Kit (Applied Biosystems, USA) according to the instructions of the manufacturer. The relative mRNA expression levels were calculated by the 2−∆∆CT method, using GADPH as an internal control for normalization [20]. The mean mRNA level of the mock-treated group was set at 1.00.
Indirect immunofluorescence assay (IFA)
The IFA was performed to confirm the inhibitory effects of LiCl on PPV replication. After washing with PBS, the ST cells from the PPV replication stage assay that had been treated with nontoxic concentrations of LiCl after allowing virus entry were fixed with 4 % paraformaldehyde in PBS for 15 min and were then permeabilized with 0.2 % Triton X-100 in PBS for 10 min. After washing, the ST cells were incubated with rabbit anti-PPV antibody (1:200) for 1 h. Subsequently, rhodamine-conjugated goat anti-rabbit IgG (1:500) (Zhongshan, China) was used as secondary antibody. After washing three times with PBS, nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) was performed for reference protein expression according to instructions of the manufacturer (Invitrogen, Carlsbad, CA, USA). After washing, fluorescence was observed under a Leica DMI4000 B confocal microscope (Leica, Wetzlar, Germany).
Statistical analysis
All experiments were performed in triplicate, and the data are presented as the mean ± standard deviation (SD). Student’s t-test was performed to compare sets of data. Differences with a two-tailed P-value less than 0.05 were considered significant.
Results
Nontoxic concentrations of LiCl
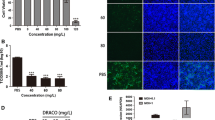
Cytotoxicity assays were performed according ti the instructions of the manufacturer of CCK8. The relative cell viability was above 90 % after treatment with LiCl concentrations of 10, 20, 25, and 30 mM, whereas the viability was under 50 % after treatment with LiCl concentrations of 50, 80, and 100 mM (Fig. 1).
The cytotoxic effect of LiCl treatment on ST cells. The cells were treated with 0, 10, 20, 25, 30, 50 or 100 mM LiCl for 24 h. Relative cell viability was determined by CCK8 assay and normalized to the value of the 0 mM group (set at 100 %). Data are expressed as the mean ± S.D. of three independent experiments
It was necessary to verify that the concentration of LiCl was nontoxic to the cells to ensure that the results of the experiments were unaffected by the drug. A LiCl concentration under the 50 % cytostatic concentration (CC50), the concentration that inhibited the proliferation of exponentially growing cells by 50 %, was defined as a non-toxic concentration. At a concentration of 30 mM LiCl, which is below the CC50, value, no difference of cell morphology compared with mock-treated cells was observed (data not shown), and this concentration was therefore chosen as the maximum concentration of LiCl for the antiviral assays.
No effect on PPV attachment and entry
Assays were performed to evaluate the effects of LiCl on PPV attachment to and entry into cells. For the viral attachment assays, the PPV titers of ST cells treated with 10, 20, 25, and 30 mM LiCl and mock-treated ST cells were all approximately 5 (Fig. 2A). The fact the significant differences in viral titers between drug-treated and mock-treated ST cells were not observed in viral attachment assays indicated that LiCl had no effect on the attachment of PPV to ST cells. Similarly, in the viral entry assays, the PPV titers of cells treated with 10, 20, 25, and 30 mM LiCl and mock-treated ST cells were all also approximately 5 (Fig. 2B). The fact that significant differences in viral titers between drug-treated and mock-treated ST cells were not observed in viral entry assays indicated that LiCl had no effect on the PPV entry into ST cells.
The virus load in ST cells treated with different concentrations LiCl at different stages of the viral cycle. A. Viral titers of cells treated with the drug at the viral attachment stage. B. Viral titers of cells treated with the drug at the viral entry stage. C. Viral titers of cells treated with the drug at the viral replication stage. D. Virus load of cells treated with the drug at the viral replication stage as determined by real-time PCR (*P < 0.05; **P < 0.01; ***P < 0.001)
At 4 °C, the viruses attach to cell receptors but do not enter the cell until the temperature is raised to 37 °C [21–23]. Based on these properties, this study was designed to determine whether LiCl inhibits PPV attachment and entry.
Effect on replication of PPV
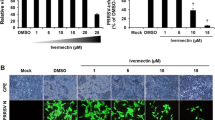
Viral replication assays were performed to evaluate the effect of LiCl on the replication of PPV in ST cells. The mean viral titers of mock-treated ST cells and cells treated with 5, 10, 15, 20, 25, and 30 mM LiCl were 5.83, 4.62, 3.58, 3.18, 2.41, and 2.09, respectively; the PPV titers decreased in a dose-dependent manner in ST cells treated with LiCl (Fig. 2C). To confirm the inhibitory effect of LiCl, a real-time PCR targeting the NS1 gene of PPV and an IFA for detection of the PPV protein load were performed. For the real-time PCR, the mean relative mRNA load of mock-treated ST cells and ST cells treated with 5, 10, 15, 20, 25, and 30 mM LiCl were 100, 75, 20, 3.33, 3.67, and 4.0 (with mock-treated cells set at 100), respectively (Fig. 2D). For the IFA, none of the drug-treated ST cells generated strong fluorescent signals 48 h after infection. The fluorescent signals declined when cells were treated with 15 mM or 30 mM LiCl. No fluorescent signals were detected for the mock-treated cells (no PPV and no drug; Fig. 3).
Inhibitory effect on PPV replication at early stages
To determine which stage of PPV replication was affected by LiCl, the time course of PPV replication was examined after addition of the drug. After the PPVs entered the ST cells, cells were treated with 30 mM LiCl at different times postinfection. The viral titers were determined after the ST cells had grown for 24 h. In contrast to the control, the replication of the virus was significantly reduced after addition of the drug at 0, 1, 3, 6, 9 and 12 h postinfection, but no further significant reductions occurred at 14, 18, and 24 h postinfection (Fig. 4). Thus, the inhibitory effect of LiCl on PPV replication occurred primarily in the early stages.
Time course of PPV replication in ST cells with LiCl treatment. ST cells were infected with PPV (MOI = 0.1), followed by treatment with 30 mM LiCl at the indicated time (hpi). Virus titers were determined at 24 hpi. “-” indicates that the cells that were not treated with LiCl. Values represent the mean ± standard deviation for three independent experiments. The asterisks indicate significant differences between mock-treated and drug-treated cells (*P < 0.05; ***P < 0.001)
Discussion
A previous study demonstrated that LiCl had an antiviral effect on herpes simplex virus when used at concentrations ranging from 1 to 10 mM [24]. Furthermore, LiCl at different concentrations showed antiviral effects on infection of cells by infectious bronchitis virus (IBV), an avian coronavirus, and transmissible gastroenteritis virus (TGEV). However, inhibition was not observed with influenza viruses or the virus causing encephalomyocarditis [25].
In this study, the antiviral effect of LiCl occurred in the early phases of PPV replication. Treatment with LiCl concentrations up to 30 mM showed no significant toxicity to ST cells. At this concentration, no differences in cell morphology were found between LiCl-treated ST cells and mock-treated ST cells (data not shown). To investigate which phase of PPV replication was sensitive to LiCl treatment, ST cells were exposed to LiCl at several points in the viral life cycle. LiCl had no effect on PPV attachment or entry into ST cells, which demonstrated that it did not directly affect the attachment of the virus to the cell receptor or the passage of viruses into cells.
The inhibitory effect of LiCl on PPV replication occurred primarily at the early stages. After being infected with PPV at 37 °C for 1 h to allow virus entry, ST cells were treated with a series of nontoxic concentrations of LiCl. The viral load in ST cell lysates after cultivation at 37 °C for 24 h was measured to evaluate the replication of the PPV in the ST cells. Real-time PCR and IFA results clearly demonstrated that inhibition of PPV replication by LiCl was dose-dependent. Li et al. reported that PPV infection was inhibited at the attachment stage, and even after this stage, with diammonium glycyrrhizinate [26]. Therefore, different drugs can inhibit the same virus by different mechanisms.
In conclusion, inhibition of PPV replication in ST cells by LiCl occurs in a dose-dependent manner in the early phase of viral replication. These results indicate that LiCl has potential as an effective anti-PPV drug. Further studies will be required to explore the mechanism of the antiviral effect of LiCl on PPV infection in vivo.
References
van Leengoed LA, Vos J, Gruys E, Rondhuis P, Brand A (1983) Porcine Parvovirus infection: review and diagnosis in a sow herd with reproductive failure. Vet Q 5(3):131–141. doi:10.1080/01652176.1983.9693887
Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM (1999) Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol 121(1):1–11. doi:10.1053/jcpa.1998.0295
Ellis JA, Bratanich A, Clark EG, Allan G, Meehan B, Haines DM, Harding J, West KH, Krakowka S, Konoby C, Hassard L, Martin K, McNeilly F (2000) Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J Vet Diagn Invest 12(1):21–27
Mahnel H, Mayr A, Bibrack B (1966) Multiplication of swine fever virus with cytopathogenic effect in piglet testis culture. J Vet Med Ser B 13(3):250–259
Cartwright SF, Lucas M, Huck RA (1969) A small haemagglutinating porcine DNA virus. I. Isolation and properties. J Comp Pathol 79(3):371–377
Cui J, Wang X, Ren Y, Cui S, Li G, Ren X (2012) Genome sequence of Chinese porcine parvovirus strain PPV2010. J Virol 86(4):2379. doi:10.1128/JVI.06852-11
Mengeling WL (1972) Porcine parvovirus: properties and prevalence of a strain isolated in the United States. Am J Vet Res 33(11):2239–2248
Wu R, Wen Y, Huang X, Wen X, Yan Q, Huang Y, Ma X, Cao S (2014) First complete genomic characterization of a porcine parvovirus 5 isolate from China. Arch Virol 159(6):1533–1536. doi:10.1007/s00705-013-1948-4
Cheung AK, Wu G, Wang D, Bayles DO, Lager KM, Vincent AL (2010) Identification and molecular cloning of a novel porcine parvovirus. Arch Virol 155(5):801–806. doi:10.1007/s00705-010-0646-8
Zeeuw EJ, Leinecker N, Herwig V, Selbitz HJ, Truyen U (2007) Study of the virulence and cross-neutralization capability of recent porcine parvovirus field isolates and vaccine viruses in experimentally infected pregnant gilts. J Gen Virol 88(Pt 2):420–427. doi:10.1099/vir.0.82302-0
Jozwik A, Manteufel J, Selbitz HJ, Truyen U (2009) Vaccination against porcine parvovirus protects against disease, but does not prevent infection and virus shedding after challenge infection with a heterologous virus strain. J Gen Virol 90(Pt 10):2437–2441. doi:10.1099/vir.0.012054-0
Manji HK, Potter WZ, Lenox RH (1995) Signal transduction pathways. Molecular targets for lithium’s actions. Arch Gen Psychiatry 52(7):531–543
Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J (1997) Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett 411(2–3):183–188
Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA (2007) Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4(7):e249. doi:10.1371/journal.pmed.0040249
Lucas KC, Hart DA, Becker RW (2010) Porcine proximal tubular cells (LLC-PK1) are able to tolerate high levels of lithium chloride in vitro: assessment of the influence of 1-20 mM LiCl on cell death and alterations in cell biology and biochemistry. Cell Biol Int 34(2):225–233. doi:10.1042/CBI20090042
Harrison SM, Tarpey I, Rothwell L, Kaiser P, Hiscox JA (2007) Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathol 36(2):109–114. doi:10.1080/03079450601156083
Li J, Yin J, Sui X, Li G, Ren X (2009) Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol 38(3):215–221. doi:10.1080/03079450902912184
Ren X, Meng F, Yin J, Li G, Li X, Wang C, Herrler G (2011) Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PLOS One 6(5):e18669. doi:10.1371/journal.pone.0018669
Boubaker Elandalousi R, Mekni Toujani M, Kaabi B, Larbi I, Diouani M, Gharbi M, Akkari H, Chir FB, Ghram A (2014) Non-cytotoxic Thymus capitata extracts prevent Bovine herpesvirus-1 infection in cell cultures. BMC Vet Res 10(1):231. doi:10.1186/s12917-014-0231-6
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Jones JC, Turpin EA, Bultmann H, Brandt CR, Schultz-Cherry S (2006) Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol 80(24):11960–11967
Hahon N, Booth JA, Eckert HL (1973) Cell attachment and penetration by influenza virus. Infect Immun 7(3):341–351
Ramalho-Santos J, Nir S, Duzgunes N, de Carvalho AP, de Lima Mda C (1993) A common mechanism for influenza virus fusion activity and inactivation. Biochemistry 32(11):2771–2779
Cernescu C, Popescu L, Constantinescu S, Cernescu S (1988) Antiviral effect of lithium chloride. Virologie 39(2):93–101
Skinner G, Hartley C, Buchan A, Harper L, Gallimore P (1980) The effect of lithium chloride on the replication of Herpes simplex virus. Med Microbiol Immunol 168(2):139–148
Li P, Zou H, Ren Y, Zarlenga DS, Ren X (2014) Antiviral effect of diammonium glycyrrhizinate on cell infection by porcine parvovirus. Curr Microbiol 69(1):82–87. doi:10.1007/s00284-014-0540-9
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31372373 to JS), the Natural Science Foundation of Guangdong Province, China (S2013010016750 to JS), Specialized Research Fund for the Doctoral Program of Higher Education, China (20134404110023 to JS), and the Key Program for Scientific and Technological Innovations of Higher Education Institutes in Guangdong Province (cxzd117).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Chen, H. Yan, and H. Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Y., Yan, H., Zheng, H. et al. Antiviral effect of lithium chloride on infection of cells by porcine parvovirus. Arch Virol 160, 1015–1020 (2015). https://doi.org/10.1007/s00705-015-2352-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2352-z