Abstract
A field experiment was carried out in plant litter decomposition at three sites of the Balaton System (Balaton — Kis Balaton wetland — Zala Mouth) differing in their environment type during winter 2019/2020. The largest freshwater shallow lake in Central Europe (Carpathian Basin) is the Balaton, with a surface area of about 600 km2 and an average depth of 3.25 m. Right around the lake, a nutrient filtering system, the Kis-Balaton wetland, is functioning to avoid water deterioration and eutrophication. The aim of the study was to investigate crop-weather relations in two sample species, the widely distributed native P. australis and the allied S. canadensis incubated beneath the water using leaf-bag technique to characterise plant organ decomposition. Based on our results, the most consistent meteorological variable regarding decomposition process was global radiation (r = − 0.62* to − 0.91**; r: correlation coefficient; * and ** mean that correlations are significant at the 0.05 and 0.01 levels), in each treatment. In modelling the decomposition process, out of eight meteorological variables, only the daily mean air temperatures and humidity were excluded from regression equations. On dominatingly windy days, with the increase in water temperature of the Zala Mouth, the sensitivity of the decomposition of S. canadensis litter tended to decrease as compared to P. australis. The remaining litter masses were in a Kis-Balaton > Balaton > Zala order, contrasting the water temperature gradient that decreased from the Zala to the Kis-Balaton wetland under wind-dominated conditions. Considering all sampling places in three aquatic ecosystems, there was a 2.2 and a 2.7% daily mean detritus mass loss in P. australis and S. canadensis, respectively. We concluded that the invasive S. canadensis litter decomposed more quickly than those of native P. australis, irrespective to sampling site. Increase in winter water temperature significantly promoted the litter decomposition of both plant species. The originality of the study is that it quantifies the litter decomposition for an Eastern European wetland, during wintertime.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The warming of the climate has been confirmed on almost every level from global to local scales (IPCC 2021) even in the Carpathian Basin. According to regional climate models of RegCM and Aladin, annual mean air temperature increases by 1–2 °C and 3–5 °C by the periods 2021–2050 and 2071–2100, respectively, were projected by Bede-Fazekas and Szabó (2019). At the same time, for winter periods 1.1–1.3 °C and 2.5–2.9 °C, warming was projected by Bartholy and Pongrácz (2019) for Hungary. At Keszthely, a precipitation decline of 0.2–0.7 mm/year was observed between 1901 and 2000 in an autocorrelated Mann–Kendall trend test (Kocsis et al. 2020).
The Carpathian Basin occupying a transition region in Europe is probably one of the most sensitive places regarding impacts of global warming (Torma et al. 2010). Whilst the climatic projections for different regions of Hungary may vary temporally, the sensitivity remains the same due to the special pool-type geographical position of Hungary (Anda and Soós 2016).
Lake Balaton is the largest shallow lake in Central Europe with a surface area of 600 km2 and an average depth of 3.25 m considered to be 17,000–19,000 years old (Cserny and Nagy-Bodor 2000). The lake’s watershed area is about 5180 km2. As the last overall publication dealing with climate of Lake Balaton was published by Béll and Takács 1974, and because of missing permanent measuring network above the lake, most authors obtain meteorological dataset from two permanent meteorological stations with long-term observations (Siófok and Keszthely) located on the lakeshore. Based on Keszthely data (1871–2014), the climate around the lake is temperate continental (after Koppen-Geiger classification Cfb) with an annual long-term mean air temperature, Ta of 10.5 °C with a monthly minimum of − 1.1 °C during January and a maximum of 21.1 °C in July (Anda et al. 2017). The warm and sunny summers from June to September are the best time to visit the lakeshore for tourists. The mean annual precipitation, PR, is 673.3 ± 137.9 mm with large monthly variation between 32.7 and 76.1 mm in the driest January and wettest July, respectively. The PR at Kis-Balaton wetland, KBW shows different distribution concentrating between May and July, contributing to about half of the highly PR showing variable spatial and temporal occurrence due to location in the Carpathian Basin (Anda et al. 2017). In winter, the lake may be covered with a sheet of ice, sometimes thicker than 0.5 m. Van Straten and Herodek (1982) revealed that “cold”-type organisms in the Balaton were active when water temperature, Tw < 10 °C, and when Tw > 15 °C, the “warm” type organisms became significant. Between the two Tw limits (10 °C > Tw > 15 °C), a transition period regarding the composition of the ecosystem was classified.
The lake is mainly dependent on precipitation for water input, with most important surface water of River Zala. As long as Zala River, a permanent streamflow, provides the largest inflow of water, the canalized Sió is the only (artificial) outflow. Majority of the water losses is due to evaporation —mostly depending on air temperatures— and transpiration playing a minor role due to low coastal canopy cover, in the long term. In the twenty-first century, increasing temperatures are estimated to reduce in long-term runoff exceeding 50% for the Zala watershed (Csáki et al. 2018). Modification in climate connected mouths, such as Ta and varied PR patterns, may influence the wetland’s hydrological cycle (Gnjato et al. 2021). The high dependence of Lake Balaton on meteorological conditions even in the future and the significant declining precipitation trend together with rising temperature trends may raise a risk situation leading to decreasing lake water levels. Low water levels induce increased nutrient concentrations enhancing eutrophication and algae growth (Soja et al. 2013). It is important to note that trophic state of the lake has changed between meso- and eutrophic ones only during the last Millennia (Korponai et al. 2011). Although oligotrophic trends were detected temporally, the lake water quality is still considered to be mesotrophic (Istvánovics et al. 2007). The water’s energy-mass cycle is basically supported by the primary production of the planktonic algae, sharply indicating the quality of the water (Vörös and Somogyi 2009). To mitigate the nutrient’s load and to keep a constant water level on the basis of natural Kis-Balaton wetland, the Kis-Balaton Water Protection System was created in the past century, to keep a standard water level. In the area of the KBW, severe microclimate modifications are experienced in the second half of the twenty-first century. Balaton and the surrounding KBW are strictly protected areas under the Ramsar Convention. The wetland cover of about 5–8% of the world’s land surface (Mitsch et al. 2013) constitute 20–30% of the total global carbon pool and play a crucial role in nutrient cycles and climate change mitigation (Dang et al. 2021a).
Increasing drought in the past decades highlighted the vulnerability of wetland ecosystems to natural climate variability and to the potential impacts of global climate change. Quijano-Baron et al. (2022) concluded that wetlands are expected to reduce flooding frequency and increase the severity of droughts that affect plant species distribution and community structure. The sensitivity of wetlands toward fluctuation in existing water level plays a decisive role in the distribution of dominant plant species. Changes in the zonation of wetland plants are the result of their adaptation to total flood and dry periods (Sorrell et al. 2000).
Phragmites australis L. (P. australis) is one of the most widespread wetland macrophytes around Lake Balaton. The area with P. australis cover is estimated to be larger than 1000 ha. In the KBW, the P. australis covered area is about two times larger than around Lake Balaton. The role of P. australis is purifying the water of the Zala Mouth by retaining nutrients. The Zala Mouth is the primary water source of Lake Balaton, carrying about half of its water and nutrient inputs. The role of KBW is to increase the residence time of the mouth’s water before entering the lake at Keszthely-Bay. The relatively low water depth of our sampling points (Lake Balaton, Kis-Balaton wetland, KBW and Zala Mouth) made the study location particularly sensitive to weather conditions. Along with the changes in climate conditions, the modified water table depth might trigger changes in vegetation composition and coverage of KBW, giving advantage for Solidago canadensis L. (S. canadensis) in competition with P. australis. Based on previous observations, these negative changes in the vegetation composition of KBW are likely to go on in the next decades. In KBW, goldenrod as an invasive species had a few per cent, but annually increasing cover between 1997 and 2012 (Anda et al. 2015). This goldenrod related statement was also strengthened by Anda et al. 2016.
Litter decomposition is determined by the interaction between plant litter quality and consumer organisms, both controlled by the environment (Makkonen et al. 2021) along with climate variables. Correa-Araneda et al. (2020) concluded that climate is the predominant factor among the environmental variables controlling litter decomposition. Júnior et al. (2020) and Amani et al (2019) called attention to difficulties in the prediction of Ta impacts on litter decomposition due to the presence of other factors (microbial activity, invertebrate detritivore density, litter quality), which may influence how decomposition responds to weather modification. Ta influences the living conditions, the existence of microbes and other soil fauna. Kirschbaum (2000) and Lloyd and Taylor (1994) found litter decomposition to be more sensitive to Ta than the primary production. Hobbie (1996) considered the Ta as a primary determiner of decomposition rate. Bölscher et al. (2020) found increased fungi to bacteria ration due to high Ta and PR facilitating soil acidification. More intense litter decomposition related to increased lignin content tended to be under higher annual mean Ta (Thevenot et al. 2010). A positive Ta decomposition relationship, with faster decomposition in tropical compared with temperate streams, was obtained from the work of Ferreira and Guérold (2017). Others found just the opposite due to favourable cool, well‐aerated conditions, which were probably preferred by aquatic hyphomycetes (Ferreira et al. 2012). Zhang et al. (2019) summed that no consistent patterns have emerged regarding litter decomposition in streams among different temperature/climate zones. However, Cornwell et al. (2008) revealed that litter quality (local interspecific variation) had stronger effects on decomposition than climate variables. Accounting the joint effect of global warming and decomposition trait aspects allows a better understanding of the background mechanisms of climate change shifts on ecosystem functioning (Migliorini and Romero 2020).
While the sensitivity of decomposition to Ta and PR has been emphasized in previous studies (Bölscher et al. 2020), hardly enough publications have focused on plant litter decomposition sensitivity to other impacting meteorological variables such as solar radiation or extreme air and water temperatures. Understanding the response of litter decomposition to energy source and other seldom studied meteorological variables, including Tw is one of our aims in order to predict the response of ecosystems to climate change. In most studies, the climatic response of decomposition is usually determined in laboratories, based on seasonal/monthly mean Ta, which is not equivalent to variation in daily mean or extreme Ta sensed in “natural” field conditions. The commonly measured meteorological variables (Ta, PR) related to decomposition were supplemented and analysed — solar radiation, (G), maximum (Tmax) and minimum (Tmin) air temperatures, daily mean water temperature (Tw), daily mean relative humidity (RH), daily mean wind speed (u) — in this study. Three sampling sites with different aquatic environments forming a single entity were included in the investigation: Lake Balaton, its purifying system the KBW and Zala Mouth, the primary water source of the lake. Unlike most publications, three water bodies — lake, river mouth, wetland — were included in the experiment, but in a different way than of the other usage. The second purpose of the study was that the invasive S. canadensis decomposition rates are different than that of the native P. australis one, due to the varied environment of three sampling points. There are some differences in Tw among three sampling sites related to their hydrological positions. There are plenty of data on the use of decomposition coefficients kexp, kT and Q10, but they were applied separately. The other objective of this work was to compare different decomposition rates using three different sampling sites in the Balaton system.
Because some of benchmark publications connected to decomposition were mostly restricted in one season/year, we also limited ourselves to winter. Among others, the following publication’s observation lengths were similar to that of this study (Lee and Bukaveckas (2002): 60 days; Bertoli et al. (2016): 45 days; Álvarez and Bécares (2006): 90 days; Jaques and Pinto (1997): 140 days)). In accordance with the observations time of Bertoli et al. (2016), the duration (98 days) of this experiment was chosen to limit analysis at wintertime level and in order to allow comparisons among wintertime results.
To provide appropriate adaptation strategies for the protection and conservation of native ecosystems, it is essential to follow the occurrence of all the species of natural ecosystems. The importance of this is underlined by Dang et al. (2021b) , who mentioned that ensemble modelling approaches are effective for predicting climate suitability for native species only. The used model approaches have not yet been applied to invasive species distribution.
2 Materials and methods
2.1 Study location and some water quality parameters
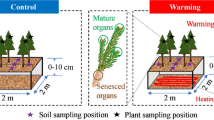
The study was conducted during the winter period of 2019/2020 at three different sampling sites (Lake Balaton (latitude 46° 74′ N; longitude 17° 28′ E; elevation 101 m above sea level), KBW (latitude 46° 65′ N; longitude 17° 19′ E; elevation 106 m above sea level) and the Zala Mouth (latitude 46° 65′; altitude 17° 20′; elevation 106 m above sea level)) to determine the decomposition rate of P. australis (rhizome, stem and leaf) and S. canadensis (stem and leaf) for plant components (Fig. 1).
Location of the study with three sampling sites near and in the Lake Balaton, Hungary. Geographical positions were included in Sect. 2
Conductivity and pH were measured in situ, at the same time as samples were taken, using Adwa AD111 and AD310 field equipment. In the laboratory, a Lovibond MultiDirect (type 0,913,462) spectrophotometer was applied to determine ammonium (NH4+ mg L−1) and phosphate (PO43− mg L−1) concentrations of different water bodies at each sampling time to separate original water properties from that of released nutrient during decay. Biological oxygen demand (BOD5) was measured using the standard of MSZ ISO 5813.
Irrespective to study sites, based on on-site macrophyte composition and cover observations, the dominant emergent macrophytes were P. australis, cattail (Typha angustifolia, Typha latifolia) and sedge (Carex acutiformis, Carex elata, Carex riparia) accounting for about 70% of canopy cover. Other minor crop species, such as shrubs (Salix cinerea, Salix alba), tree patches (Salix fragilis, Alnus glutinosa, Populus tremula) or grassland (dominant species: Festuca rupicola, Arrhenatherum elatior, Alopecurus pratensis) were also identified with much less than 10% cover (Anda et al. 2015). In addition to the above species, S. canadensis was also present in every sampling area.
2.2 Decomposition of plant litter
To detect the decomposition rate, the commonly used and accepted method of leaf litter bags were applied (Bärlocher et al. 2020). The litter bags were placed into the water on 9 December 2019. Along the sampling sites, leaf, stem and rhizome litters of P. australis and S. canadensis were collected and brought to the laboratory in October 2019. Later, the plant materials were air dried and stored at room temperature (21–22 °C) and low humidity (about 40%) to constant weight and cut into 4–6-cm-long sections, except for the S. canadensis leaf. Dried leaf, stem and rhizome litters were applied to reach as uniform conditions as possible.
The mesh sizes (15 cm × 15 cm polyethylene bags with 3 mm) of 405 litter bags (three sampling places, nine temporal sampling events, two to three plant tissues in two species, three replications each plant tissue) were filled with 10-g dry weight. The bags were randomly attached to a plastic compartment with bricks placed in the centre of the compartment. Bags were randomized and separated to avoid spatial confounds. The litter samples were placed into the water of the sampling sites at about 0.3 m below the water surface in the littoral zone to ensure that the bags will constantly remain underwater. At each sampling time, three bags were retrieved from each sampling place in separate plastic boxes on days 1, 2, 3, 7, 14, 21, 42, 70 and 98 after placing the litter bags into the water. After sample takings, the litter bags were transferred to the laboratory, and they were cleaned and dried until constant weight at room temperature and low humidity.
We designed this experiment with the goal to study spatial and temporal influences of the decomposition process to compare results obtained in a native and an invasive plant, at wintertime. The composition in microorganisms was excluded from the study. There was no significant difference in the density of macroinvertebrate shredders between the sampling points (Simon et al. 2018, 2019).
2.3 Meteorological elements
Meteorological variables were recorded at the Agrometeorological Research Station of Keszthely (latitude 46° 44′ N, longitude 17° 14′ E, elevation 124 m above sea level) by a QLC-50 (Vaisala, Helsinki, Finland) fitted with a CM-3 pyranometer (Kipp & Zonen Corp., Delft, The Netherlands). A CM-3 pyranometer sensor (Kipp & Zonen Corp., Delft, the Netherlands) was installed to QLC-50 automatic station to measure global radiation, G [W m−2], because of its importance for the whole climate system as a primary energy source impacting other meteorological variables and environmental processes. This standard station belongs to the Hungarian National Meteorological Network operated by the Hungarian Meteorological Service (OMSZ). The combined air temperature and humidity sensors were placed at a standard height 2 m above the soil surface. Signals from Ta, RH, u, PR and G were collected every 2 s, and 10-min means were logged by the QLC-50 station. The height of the anemometer was 10.5 m (Anda et al. 2015). In situ Tw data were recorded at 10-min intervals and integrated to daily means (Delta Ohm HD-226–1).
2.4 Estimation of decomposition constants (k exp, k T) and temperature sensitivity coefficient (Q 10) based on Bärlocher (Bärlocher et al. 2020)
The process of plant litter decay was typically described by first-order kinetics, in which temporal loss of litter mass at a given point is assumed to be proportional to the present litter mass.
Decomposition rates were estimated using an exponential model, in which the decaying rate follows the equation:
where m (g) is the mass loss as a proportion of initial mass, t the time in days after the initial exposure, and kexp (day−1) is the decomposition constant. This relationship is independent on temperatures (Ta, Tw).
The first-order decay model was expanded assuming a linear temperature (Tw) dependency of the overall decay rate:
where kT (day−1) is the temperature-normalized decomposition rate coefficient. Set TR = 10 °C as Bärlocher et al. (2020) suggested, for ease of comparison with other temperature-based models.
To compute the temperature sensitivity coefficient Q10, in which decomposition rate is increased when temperature is raised by 10 °C, an exponential instead of a linear relationship between temperature and decomposition rate was assumed:
Using Eq. 3, the Q10 values were used to determine the constant c. In the next step, the Q10 was computed as follows:
2.5 Statistics
Correlation analysis and multiple stepwise regression analysis between meteorological elements (daily means of Ta and Tw; air temperature extremes Tmin and Tmax; G; RH; u, PR) and measured decomposition rates in P. australis (rhizome, stem and leaf) and S. canadensis (stem and leaf) were carried out. Three-way ANOVA was performed applying SPSS software, version 17.0, to estimate the impact of sampling sites (S), crop species (C) and plant organs (O) on the remained litter and decomposition coefficients, kexp day−1 and kT day−1. The used model comprised all main effects and all two- and three-way interactions followed by Student’s t test or Tukey’s HSD post hoc test. Independent-samples t test was used to compare all variations of treatments (S, C, O) for k day−1. Critical value of significance was set to 0.05.
3 Results and discussion
3.1 The water quality of the three sampling sites and the weather conditions
During the study period in winter 2019/2020, the BOD5 increased from 1.7 (Balaton) to 6.8 mg L−1 (Zala transporting suspended solids) and the pH from 8.0 to 8.4 (the most favourable regarding decomposition) in the observation sites (Table 1). High conductivity values were displayed (680–807 µS cm−1) with low nutrient concentrations (0.2–0.5 NH4–N mg L−1 and 0.6–0.8 PO4–P mg L−1), irrespective to study site. Significant differences were not found in the physical and chemical properties of the water at the sampling places, although the meteorological variables might have played a major role in the variance of the litter decomposition of the studied plants.
The location of the study, the Lake Balaton and KBW, positioned in the Carpathian Basin has variable inter- and intra-annual climate conditions. The climate norms (1981–2010) monitored at the Agrometeorological Research Station of Keszthely (Table 2; Fig. 1) were used in the weather characterization of the observation period (9 December 2019–16 March 2020). The sampling period’s mean Ta was unusually warm with an average of 3.9 °C, at about fourfold higher (p < 0.001) than that of the long-term mean Ta of Keszthely. At the same time, within the study period, the monthly mean Ta in January (2020) was 4.9 and 6 °C colder than the monthly mean Ta in December and February, respectively. Close to normal Ta was only detected in March 2020. The warm weather was associated with an 18.3% higher PR sum (p = 0.603) as compared to long-term PR total. The monthly PR pattern of the study period was unevenly distributed. December (2019) received 34.5 mm more while January (2020) received 16.9 mm less monthly total PR than the climate norm. Daily PR amounts in December (2019) were more frequent and of larger magnitude than that of the long-term PR sums. Almost all studied months were hotter and wetter than the long-term means for 1981–2010.
The winter patterns of daily mean Tw were similar at each sampling site; however, the size of Tw varied among observation areas (Fig. 2). During the study period in winter, 2019/2020, the average Tw varied from 3.1 (KBW) to 6.0 °C (Balaton). Temporal patterns and treatment differences in Tw agreed well with varying weather conditions; calm weather from 22 to 53 days after placing decomposition samples exhibited a high degree of spatial variation in Tw. In this winter period, Tw of Lake Balaton with the largest water body was the highest (4.7 °C). At the same time period, declines in Tw of the Zala Mouth and KBW were 3.0 and 4.8 °C, respectively, compared to Balaton. In the beginning (from 1 to 21 days after sample placing) and after a calm period (from 54 days until the end of the study), the windy and warm weather (daily mean u > 1 m s−1, daily average Ta > 2 °C) equalized Tw values between the three sampling places. Although, variability in mean Tw during two windy periods (6.6 ± 1.3, 4.7 ± 2.6 and 6.9 ± 2.9 °C in Balaton, KBW, Zala Mouth, respectively) exceeded those observed on calm days (Balaton: 4.7 ± 1.1 °C, KBW: − 0.1 ± 0.3 °C, Zala Mouth: 1.7 ± 0.9 °C), irrespective to sampling site.
3.2 Remaining litter and litter mass loss
Litter mass remaining declined exponentially over the study period (Fig. 3), and after 98 days, it varied between 60.2 and 91.7% of initial mass in Balaton, 67.8 and 92.9% in KBW and 59.2 and 90.2% in the Zala estuary for P. australis (including rhizomes). For S. canadensis, less variability in remaining litter mass was detected ranging from 61.0 (Zala Mouth) to 85.5% (KBW) of initial mass.
Daily mean P. australis litter mass loss ranged from 0.0073 (stem in KBW) to 0.0416 g days−1 (rhizome in Zala Mouth), while in S. canadensis, these daily litter mass loss values were between 0.0023 (KBW, leaf) and 0.0398 g days−1 (Zala, leaf).
The results of the three-way ANOVA suggested that the remaining litter mass was influenced by sampling sites, S (p = 0.002), crop species, C (p < 0.001) and plant organs, O (p < 0.001) (Table 3), with insignificant interactions between the three factors (p = 0.425–0.879). A significant interaction was found between sampling sites, plant species and tissues (p < 0.001) suggesting that effects in litter species and studied plants were site dependent.
Irrespective to study location, the remaining litters of invader S. canadensis were 8.2 (leaf, KBW) — 27.9% (leaf, Balaton) lower than that of in native P. australis. Stem decompositions were comparable to leaf ones (between 8.3 and 17.3%). This result has not supported the assumption of the native plant’s advantage based on the local decomposer communities’ specialization to the native litter types being faster than to the litter of invasive plants (Dekanová et al. 2021). Considering all sampling places in three aquatic ecosystems, a daily mean detritus mass loss of 2.20 and 2.68% was found in P. australis and S. canadensis, respectively. Our study result concerning P. australis mass loss was close to that of the 1.57% in the Danube Delta which is pooled from four wetland ecosystems (Sangiorgio et al. 2008). The remaining litter masses decrease in the order KBW > Balaton > Zala, counter the Tw gradient that decreased from Zala to KBW. The 12.6, 12.7 and 12.7% declines in the remaining mass for P. australis leaf, stem and rhizome, respectively, were obtained in Zala compared to KBW. A possible explanation for the lowest decomposition rates in KBW may be that less plant material was lost from litterbags in comparison with the Zala Mouth. The water in the KBW was almost static due to a high number of windless days during the observation. Because of the lack of turbulence, undecomposed litters were not easily loosened from litterbags, increasing the remaining mass in such “stationary” water as KBW compared to other aquatic environments (Song et al. 2021).
Observed plant processing fitted with negative exponential models in all the monitored treatments’ temporal patterns (see Fig. 3). The total percentage of P. australis detritus weight loss ranged from 0.07 (stem, KBW) to 0.42% (rhizome, Balaton) for the whole observation time. P. australis litter mass loss in the study was about half of that measured by Bertoli et al. (2016) at Isonzo delta (Italy), during spring 2011. In S. canadensis, mass loss percentages were between 0.15 (leaf, KBW) and 0.39% (leaf, Zala), over the winter 2019/2020. Tukey post hoc test revealed the lowest remaining litter mass (averaged across plant tissues) in the Zala Mouth. Regardless of the low contribution of stream/mouth macroinvertebrates to litter decomposition in Azorean streams reported by Ferreira et al. (2016), the remaining mass was the lowest in the Zala Mouth. (The author’s results were within the range reported for continental streams.) A 12.4% increase (p = 0.011) in the remaining litter mass was measured in KBW related to the Zala Mouth. Litter mass remaining in Balaton was only slightly higher (below 5%) than that obtained in the Zala, although the difference was insignificant (p = 0.630).
3.3 Decomposition coefficients (k exp and k T)
Five classes (bubbles) of kexp on Fig. 4 contain different plant tissues. The first two bubbles were for plant leaves (1: P. australis; 2: S. canadensis). The least size and variability in kexp of different sampling sites was obtained in P. australis rhizome (last bubble). Other works also presented that the content of lignin negatively correlated with the rate of litter decomposition (Huang et al. 2003). In the case of rhizome decomposition process, the high lignin content of the organ was more important than that of the sampling site. Results in the study strengthened the previous ones by Huang et al. (2021) that the lignin content of the litter may be a basic indicator of the decomposition rate of different litter types in aquatic environments. The kexp averaged across P. australis parts ranged from 0.0208 (KBW) to 0.0261 day−1 (Balaton) suggesting fast decomposition rates (k > 0.010 day−1) (Petersen and Cummins 1974), somewhat lower kexp for S. canadensis, but in fast range were between 0.0167 (KB) and 0.0254 day−1 (B) during the observation. These fast decay rates of P. australis leaves in each study site were comparable with previous ones found in the Danube Delta by Sangiorgio et al. (2008). Fast decomposition in P. australis highlighted that litter inputs to Balaton and its surroundings are effectively processed; the nutrients are probably cycled at an acceptable level.
Boxplots for exponential decomposition rate, kexp in P. australis and S. canadensis. Five bubbles were used to demonstrate similar classes for decomposition coefficients. Abbreviations in the horizontal axes are as follows: First letter means sampling sites: B — Balaton Lake; K — Kis-Balaton wetland; Z — Mouth Zala. Second letter means crop species: r — P. australis; g — S. canadensis. Third letter means plant organs: l — leaf; r — rhizome; s — stem. Means with the same letters are not significantly different among the two species, three tissues and three sampling sites
P. australis results averaged across plant tissues in the study agreed well with that of 0.028 day−1 by Pinna et al. (2004) in a mouth basin in Sardinia, Italy, during spring 2004. In the Netherlands, in a Monomictic Lake, winter kexp of 0.012 day−1 in P. australis (van Dokkum et al. 2002) was only half of that measured in this study. Similarly, findings for P. australis decomposition rates of Sangiorgio et al. (2008) differed among aquatic ecosystems, with lower values in pond compared to values in channel/mouth. Their kexp of 0.016 day−1 for P. australis blades in Danube Delta (fall 2005) was almost the same as kexp of 0.0178 day−1 in the study for P. australis blades in the Zala Mouth. The decomposition coefficient’s variability was also related to differences in plant tissues. S. canadensis kexp of 0.0017 day−1 (Dekanová et al. 2021) in an artificial pond in Slovakia concurred well with findings of the study (condensed for two plant tissues: 0.0192 day−1, KBW). The authors also recorded the faster litter decomposition of invasive plants than of native ones. The thinner and softer leaves of S. canadensis were more easily disrupted and processed than those of the native P. australis leaves.
The kT accounting Tw was also brought into existence for aquatic ecosystems’ litter decay. Estimated kexp and kT ranged from 0.0087 day−1 (P. australis leaf, KBW) to 0.0497 day−1 (P. australis rhizome, Balaton) and from 0.0177 day−1 (P. australis stem, KBW) to 0.672 day−1 (P. australis rhizome, Balaton), respectively, throughout the investigation period. Higher Tw-based kT than that of exponential kexp were determined, irrespective to treatment.
As a result, the decomposition coefficients kT highly varied in all tissues and sampling places from 0.0177 ± 0.0014 day−1 (KBW, reed stem, class “a”) to 0.6726 ± 0.0689 day−1 (Balaton, reed rhizome, class “h”) constituting separate classes from “a” to “l” (Table 4). Plant tissues of leaf, stem and rhizome (P. australis only) were pooled for each sampling site; thus, the mean kT of Balaton, KBW and Zala Mouth were 0.0253, 0.0192 and 0.0256 day−1, respectively, for the 98-day-length decomposition period (Table 4). At the same time, much higher averaged pooled kT were recorded in all study sites (Balaton: 0.3259 day−1, KBW: 0.0438 day−1, Zala Mouth: 0.1024 day−1). At species level, plant traits and the environmental conditions play a dominant role in the regulation of the decomposition process. The approach of Tw-based kT has several useful implications compared with the results of previous works by using exponential litter decomposition rates, kexp (Gregorich et al. 2016). Values of kT were refined related to kexp by accounting the site’s Tw. Cai et al. (2021) offer the use of kT to compare decay rates across different sites as it is in the study. Regarding both k of different plant organs, with the exceptions of kT in KBW and Zala, the k sizes were in the following order: rhizome > S. canadensis leaf > S. canadensis stem > P. australis leaf > P. australis stem. Both kexp (Figs. 4 and 5) and kT (Table 4) varied widely among treatments in winter 2019/2020.
ANOVA results showed that both k values differed among plant species (p < 0.001), sampling sites (p < 0.001) and litter species (p < 0.001), with significant interactions between the three factors in kexp (p < 0.001) (Tables 5 and 6). No significant effect of sampling site interacting with tissues (p = 0.110) or plant species (p = 0.153) was found in case of kT (Table 6).
Irrespective to k-type, the KBW with the shallowest water constituted the least mean k for each plant tissue. The increases in average kexp and kT in deeper Lake Balaton were 24.2% (p = 0.525) and 86.6% (p = 0.0202), respectively, as compared to KBW. In Zala Mouth, 25.2% (p = 0.477) and 57.2% (p = 0.126) increments in kexp and kT, respectively, were obtained related to KBW.
Except of P. australis leaf (p = 0.936) in the Zala, significant differences between kexp and kT ranged from 75.9 (S. canadensis stem, Zala Mouth, p = 0.002) to 92.8% (S. canadensis leaf, Balaton, p = 0.006) in Balaton and the Zala. However, irrespective to plant tissue, hardly enough variability in both k values of KBW was detected. Woodward et al. (2012) found significantly higher decomposition rates in mouths and streams due to more absorbed energy for each degree of change in air temperature than those in terrestrial ecosystems. Increased energy in the Zala Mouth during dominatingly windy days might stimulate the microbe’s metabolism, utilizing litter residues as a substrate (Gessner et al. 1999) more effectively as it happened in KBW and/or Balaton.
In the study, a linear distribution curve for kT was used as a function of kexp (Fig. 5), for the whole measuring period. As kexp versus kT were widely scattered for each sampling site, their relationship was treated separately. Both decomposition functions kexp and kT markedly decreased in KBW as also indicated by the linear equations with slopes of 14.5, 5.29 and 1.48 for Balaton, Zala Mouth and KBW, respectively. The correlation between kexp and kT (R2 = 0.96) was better in Lake Balaton than in KBW (R2 = 0.81) and the Zala (R2 = 0.87). As long as correlation was higher in Balaton, the mean RMSD in KBW was lower (0.011 day−1) than that of the RMSE of 0.048 day−1 in Balaton and 0.030 day−1 in the Zala.
During the investigation time, kexp were 20.2–54.3% of kT in KBW. In case of the Zala Mouth, kexp were 20.3–95.7% of kT. In Balaton, kexp was considerably smaller (7.2–12.9%) of kT than in KBW and/or the Zala. Examination of the k ration indicated that its size may also be linked to plant tissue. Irrespective to observation place, kexp was closer to kT for P. australis (KBW: P. australis rhizome and stem; Balaton and the Zala: P. australis leaf). Ration of kexp/kT became quite small in S. canadensis, with a minimum value of 7.2% for Balaton.
3.4 The impact of weather on litter decomposition
Litter decomposition is a multivariate process also influenced by different meteorological variables (Ferreira et al. 2015). Irrespective to sampling place and studied plant, with one exception of P. australis shoot in KBW, the most dominant meteorological variable with the highest correlation coefficient, r ranging from − 0.62* to − 0.91** was G (Table 7), related to available energy. The shoot of P. australis in KBW possessed the highest r (0.59*) for Tw. G alone explained 62.0 to 91.3% of the variability in the studied plant organ’s decomposition during winter 2019/2020. The second largest impacting variable fluctuated between the three sampling sites. With the exceptions of P. australis shoot in Balaton and KBW, the RH, integrating the impact of Ta and air humidity, was the second most relevant factor in plant tissue decomposition (0.56* ≤ r ≤ 0.74**). In the case of the Zala Mouth, the r values of Tw exceeded the r of RH ones, explaining variability in decomposition process from 61.2 to 68.6%. It is important to mention that a significant effect of Tw on the decomposition of P. australis rhizome (r = 0.82**) and S. canadensis stem (0.58*) was also observed in KBW. The rates of r for Ta characteristics were scattering around 0.2–0.4 with some exceptions of P. australis rhizome (r = 0.6*–0.70**) and S. canadensis stem (r ≤ 0.5*–0.60*) in KBW and Balaton. Based on low r values (excluding P. australis rhizome in Zala Mouth), two inefficient meteorological variables were PR and u.
As the correlation analysis suggested that apparently more meteorological variables affected decomposition rate, all measured meteorological elements were included in the multiple stepwise regression (Table 8). Projection of decomposition for studied sampling areas and plant organs was treated separately (plant: P. australis, S. canadensis, plant organs: rhizome, leaf, stem and sample sites: Lake Balaton, KBW, Zala Mouth). Adjusted coefficient of determination (R2) produced by SPSS was used for model selection. Only the best fit is presented in Table 8. Out of eight variables (G, Ta, Tmax, Tmin, Tw, RH, u, PR), only the daily mean Ta and RH were excluded from regression equations. On the basis of regression analysis, with one exception of P. australis shoot in KBW, G was included in each decomposition projection, irrespective to sampling sites and/or plant organs, making the available energy the most relevant factor for litter decomposition. The temperature extremes, Tw and PR, also affected the decomposition projection in some of the treatments (in Balaton: Tw; in KBW: Tw, Tmax, Tmin; and in Zala Mouth: Tmin, PR). Tw became an affecting variable in decomposition for Lake Balaton, with the exception of S. canadensis shoot. In KBW, there was only one plant organ, the P. australis rhizome, in which the Tw was included in the projection. In Zala Mouth, in addition to G, the projection equations were completed with the meteorological variables Tmin and PR in P. australis shoot and P. australis rhizome.
3.5 Temperature sensitivity coefficient, Q 10
Computed Q10 indicates decomposition rate increments when Tw is raised by 10 °C (Fig. 6). Irrespective to P. australis tissues, the lowest Q10 values ranging from 1.4 (rhizome) to 1.9 (leaf) were measured in Lake Balaton. Increments in Q10 for P. australis tissues ranged from 20.1 (leaf, Zala) to 37.1% (stem, Zala) as compared to Q10 of P. australis parts at Lake Balaton. In KBW and Zala, the same order of the Q10 for different P. australis tissues was stem > leaf > rhizome.
The Tw sensitivity of the two studied plants varied in different sampling sites. The Q10 in the study agreed well with the wetland’s macrophyte Q10 results of Bärlocher et al. (2020) who found them to be about 2, with the meaning that decomposition rates double when Tw is increased by 10 °C. On the other hand, Hietz (1992) called attention to the fact that Q10 has rarely been used in litter decomposition studies in aquatic environments.
In Balaton and KBW, the Tw sensitivity, the Q10 of the decomposition of S. canadensis litter was higher than the Q10 of P. australis. The mean Q10 of condensed P. australis tissues (rhizome + stem + leaf) were 1.6, 2.2 and 2.4 for Lake Balaton, KBW and the Zala Mouth, respectively, and greatly varied for different litter parts (rhizome, leaf and stem) (Fig. 6). Less variation in the average Q10 values for S. canadensis tissues (stem and leaf) were observed at each sampling site (Balaton: 2.7, KBW: 2.6 and Zala Mouth: 2.2).
Due to changes in temperature, the decomposition process of different types of litter in wetlands can modify the location’s nutrition environment which might affect the accumulation of carbon and nutrient turnover (Gao et al. 2022). Although the Q10 of S. canadensis was very similar to that in P. australis, in the Zala Mouth, it was slightly reduced compared to P. australis.
4 Conclusions
P. australis and S. canadensis litter decompositions were studied in three different sampling sites of Lake Balaton, KBW and the Zala Mouth, during winter 2019/2020. The following conclusions were drawn from the experiment over study site:
-
1.
Spatial variation was observed between sampling areas (Lake, KBW and River Mouth); therefore, we concluded that the leaf litter decomposition proceeds heterogeneously over three parts of the whole study site. The remaining mass in the leaf bags differed among observation places with the lowest remaining litter having been found in the River Zala Mouth.
-
2.
P. australis and S. canadensis processing slightly varied in the studied aquatic ecosystems. Daily decomposition rates were related to sampling areas with different environmental conditions. Litter breakdown rates were controlled rather by aquatic environment (and litter quality), and not the invasive status.
-
3.
During the study period, values of kT based on Tw were on a higher magnitude than the commonly used kexp. Values of kT seemed to be more sensitive to any changes in the decomposition process than that of the kexp. Less variability in both k values of the shallowest KBW was observed. Variability in both decomposition rates (kT and kexp) within the study areas might mostly be explained by plant features (species and plant parts) and a lesser extent by environmental conditions (meteorological variables).
-
4.
The most consistent meteorological variable regarding decomposition process was the least accessible G, irrespective of either sampling places or plant organs. Litter decomposition in the aquatic environment (near and in the Lake Balaton) was also driven by Tw. Close relationship between Tw and u was observed in and around the wetland area, during wintertime. In windy periods, the higher Tw of River Zala (no freezing during the study period) compared to the other two places favoured litter decay, which is probably attributable to higher biological activity.
-
5.
Instead of monthly (seasonal) meteorological means widely applied in decomposition experiments, daily meteorological variables should be preferred to use. Moreover, correlation between litter decay and most often used daily mean Ta and RH was not confirmed in any of the aquatic environment.
-
6.
Tw based temperature sensitivity coefficient (Q10) of the decomposition of S. canadensis litter was higher than that of P. australis, regarding each growth location. Varied Q10 of native and invasive alien crops may affect the competitive balance among wetland vegetation, stimulating the growth of invasive plants. As a result, invasive plants with more intense decomposition rates can produce immediately available nutrients. The invasive S. canadensis may become more dominant in the future as this crop is more adaptable to wetland environment than the native P. australis.
Climate change is expected to put more pressure on almost every ecosystem, particularly on wetlands. The increased frequency of time periods under modified weather condition affects the natural ecosystems, which may provide an increase in the green fractional cover of the invasive plants. For the study sites, in relation to the most extended P. australis and non-native S. canadensis, degradation in P. australis would be expected. As a result of the S. canadensis bloom, this terrestrial vegetation colonizes the site, increasingly damaging previously formed in site vegetation cover.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
All the calculations and plots have been done using Microsoft Excel and SPSS software.
References
Álvarez JA, Bécares E (2006) Seasonal decomposition of Typha latifolia in a free-water surface constructed wetland. Ecol Eng 28:99–105. https://doi.org/10.1016/j.ecoleng.2006.05.001
Amani M, Graca MAS, Ferreira V (2019) Effects of elevated atmospheric CO2 concentration and temperature on litter decomposition in streams: a meta-analysis. Hydrobiol 104:14–25. https://doi.org/10.1002/iroh.201801965
Anda A, Soós G (2016) Some physiological responses of agricultural crops to global warming. Időjárás 120(1):85–101
Anda A, Soós G, Teixeira da Silva JA, Kozma-Bognár V (2015) Regional evapotranspiration from a wetland in Central Europe; in a 16-year period without human intervention. Agric for Meteorol 205:60–72. https://doi.org/10.1016/j.agrformet.2015.02.010
Anda A, Soós G, da Teixeira SJA (2017) Leaf area index for common reed (Phragmites australis) with different water supplies in the Kis-Balaton wetland, Hungary, during two consecutive seasons (2014 and 2015). Időjárás / Quarterly Journal of the Hms 12(3):265–284
Anda A, Sujtó E, Soós G (2016) Egy új özönnövény, a magas aranyvessző párolgása a Balaton-közeli területeken (A new invasive plant, the goldenrod’s evapotranspiration nearby the Lake Balaton). Légkör: Az Országos Meteorológiai Intézet Szakmai Tájékoztatója (Announcement of the Hungarian Meteorological Service) 61:3, 93–98.
Bärlocher F, Gessner MO, Graca MAS (2020) Leaf mass loss estimated by the litter bag technique. In methods to study litter decomposition. A Practical Guide (2nd ed.) SpringerNature Switzerland AG.; Part 1. 43–51. https://doi.org/10.1007/978-3-030-30515-4_6
Bartholy J, Pongrácz R (2019) Global and regional climate change, extreme events. In: Palocz-Andresen M, Szalay D, Gosztom A, Sípos L, Taligás T (eds) International Climate Protection. Springer Verlag, Hamburg, Germany, pp 21–28
Bede-Fazekas Á, Szabó K (2019) Predicting future shift of drought tolerance zones of ornamental plants in Hungary. Időjárás 123(1):107–126. https://doi.org/10.28974/idojaras.2019.1.3
Béll B, Takács, (eds) (1974) A Balaton éghajlata (Climate of the Lake Balaton). OMSZ, Budapest
Bertoli M, Brichese G, Michielin D, Ruzic M, Vignes F, Basset A, Pizzul E (2016) Seasonal and multi-annual patterns of Phragmites australis decomposition in a wetland of the Adriatic area (Northeast Italy): a three-years analysis. Knowl Manag Aquat Ecosyst 417:14. https://doi.org/10.1051/kmae/2016001
Bölscher T, Ågrenc GI, Herrmann AM (2020) Land-use alters the temperature response of microbial carbon-use efficiency in soils – a consumption-based approach. Soil Biol Biochem 140:107639. https://doi.org/10.1016/j.soilbio.2019.107639
Cai A, Liang G, Yang W, Zhu J, Han T, Zhang W, Xu M (2021) Patterns and driving factors of litter decomposition across Chinese terrestrial ecosystems. J Clean Prod 278:123964. https://doi.org/10.1016/j.jclepro.2020.123964
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Correa-Araneda F, Tonin AM, Pérez J, Álvarez K, López-Rojo N, Díaz A, Esse C, Encina-Montoya F, Figueroa R, Cornejo A, Boyero L (2020) Extreme climate events can slow down litter breakdown in streams. Aquat Sci 82(25):1–7. https://doi.org/10.1007/s00027-020-0701-9
Cs T, Coppola E, Giorgi F, Bartholy J, Pongrácz R (2010) Validation of a high-resolution version of the regional climate model RegCM3 over the Carpathian Basin. J Hydrometeorol 12(1):84–100. https://doi.org/10.1175/2010JHM1234.1
Csáki P, Szinetár MM, Herceg A, Kalicz P, Gribovszki Z (2018) Climate change impact on the water balance – case studies in Hungarian watersheds. Időjárás 122:81–99. https://doi.org/10.28974/idojaras.2018.1.6
Cserny T, Nagy-Bodor E (2000) Limnogeological investigations on Lake Balaton. In: Gierlowski-Kordesch, E., Kelts, K. (Eds), Lake basins through space and time. AAPG Studies in Geology 46:605–618.
Dang ATN, Kumar L, Reid M, Mutanga O (2021) Fire danger assessment using geospatial modelling in Mekong delta, Vietnam: effects on wetland resources. Remote Sens Appl: Soc Environ 21:100456. https://doi.org/10.1016/j.rsase.2020.100456
Dang ATN, Kumar L, Reid M, Anh LNT (2021) Modelling the susceptibility of wetland plant species under climate change in the Mekong Delta, Vietnam. Ecol Inform 64:101358. https://doi.org/10.1016/j.ecoinf.2021b.101358
Dekanová V, Svitková I, Novikmec M, Svitok M (2021) Litter breakdown of invasive alien plant species in a pond environment: rapid decomposition of Solidago canadensis may alter resource dynamics. J. Limnol 90:125911. https://doi.org/10.1016/j.limno.2021.125911
Ferreira V, Guérold F (2017) Leaf litter decomposition as a bioassessment tool of acidification effects in streams: evidence from a field study and meta-analysis. Ecol Indic 79:382–390. https://doi.org/10.1016/j.ecolind.2017.04.044
Ferreira V, Encalada AC, Graça MAS (2012) Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshw Sci 31(1):945–962. https://doi.org/10.1899/11-062.1
Ferreira V, Chauvet E, Canhoto C (2015) Effects of experimental warming; litter species; and presence of macroinvertebrates on litter decomposition and associated decomposers in a temperate mountain stream. Can J Fish Aquat 72:206–216. https://doi.org/10.1139/cjfas-2014-0119
Ferreira V, Raposeiro PM, Pereira A, Cruz AM, Costa AC, Graca MAS, Gonçalves V (2016) Leaf litter decomposition in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions. Freshw Biol 61(5):783–799. https://doi.org/10.1111/fwb.12749
Gao S, Song Y, Song C, Wang X, Ma X, Gao J, Cheng X, Du Y (2022) Effects of temperature increase and nitrogen addition on the early litter decomposition in permafrost peatlands. Catena 209(1):105801. https://doi.org/10.1016/j.catena.2021.105801
Gessner MO, Chauvet E, Dobson MA (1999) Perspective on leaf litter breakdown in streams. Oikos 85:377–384. https://doi.org/10.2307/3546505
Gnjato S, Popov T, Adžić T, Ivanišević M, Trbić G, Bajić D (2021) Influence of climate change on mouth discharges over the Sava Mouth watershed in Bosnia and Herzegovina. Időjárás 125(3):449–462. https://doi.org/10.1007/978-3-030-03383-5_6
Gregorich EG, Janzen H, Ellert BH, Helgason BL, Qian B, Zebarth BJ et al (2016) Litter decay controlled by temperature; not soil properties; affecting future soil carbon. Glob Chang Biol 23:1725–1735. https://doi.org/10.1111/gcb.13502
Hietz P (1992) Decomposition and nutrient dynamics of reed (Phragmites australis (Cav.) Trin. ex Steud.) litter in Lake Neusiedl; Austria. Aquat Bot 43:211–230. https://doi.org/10.1016/0304-3770(92)90068-T
Hobbie E (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66(4):503–522. https://doi.org/10.2307/2963492
Huang Y, Shen Y, Zhou M, Ma R-S (2003) Decomposition of plant residue as influenced by its lignin and nitrogen. Chin J Plant Ecol 27(2):183–188. https://doi.org/10.17521/cjpe.2003.0028
Huang XL, Chen JZ, Wang D, Deng MM, Wu MY, Tong BL, Liu JL (2021) Simulated atmospheric nitrogen deposition inhibited the leaf litter decomposition of Cinnamomum migao H. W. Li in Southwest China. Sci Rep 11(1):1748. https://doi.org/10.1038/s41598-021-81458-3
IPCC Climate Change (2021) The physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds.). Cambridge University Press
Istvánovics V, Clement A, Somlyody L, Specziar A, Toth LG, Padisak J (2007) Updating water quality targets for shallow Lake Balaton (Hungary), recovering from eutrophication. Hydrobiol 581:305–318. https://doi.org/10.1007/s10750-006-0509-1
Jaques N, Pinto P (1997) Seasonal differences in the decomposition of Typha angustifolia leaves in a Mediterranean river. Limnetica 13:19–23
Júnior EDS, Martínez A, Goncalves AL, Canhoto C (2020) Combined effects of freshwater salinization and leaf traits on litter decomposition. Hydrobiol 847:3427–3435. https://doi.org/10.1007/s10750-020-04348-1
Kirschbaum MUF (2000) Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochem 48:21–51. https://doi.org/10.1023/A:1006238902976
Kocsis T, Kovács-Székely I, Anda A (2020) Homogeneity tests and non-parametric analyses of tendencies in precipitation time series in Keszthely; Western Hungary. Theor Appl Climatol 139:849–859. https://doi.org/10.1007/s00704-019-03014-4
Korponai J, Varga KA, Lengré T, Papp I, Tóth A, Braun M (2011) Paleolimnological reconstruction of the trophic state in Lake Balaton (Hungary) using Cladocera remains. Hydrobiol 676:237–248. https://doi.org/10.1007/s10750-011-0898-7
Lee AA, Bukaveckas PA (2002) Surface water nutrient concentrations and litter decomposition rates in wetlands impacted by agriculture and mining activities. Aquat Bot 74:273–285. https://doi.org/10.1016/S0304-3770(02)00128-6
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8(3):315–323. https://doi.org/10.2307/2389824
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, vanRuijven J, van Bodegom PM et al (2021) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041. https://doi.org/10.1111/j.1461-0248.2012.01826.x
Migliorini GH, Romero GQ (2020) Warming and leaf litter functional diversity; not litter quality; drive decomposition in a freshwater ecosystem. Sci Rep 10:20333. https://doi.org/10.1038/s41598-020-77382-7
Mitsch WJ, Bernal B, Nahlik AM, Mander Ü, Zhang L, Anderson CJ, Jørgensen JE, Brix H (2013) Wetlands, carbon, and climate change. Landsc Ecol 28:583–597. https://doi.org/10.1007/s10980-012-9758-8
Petersen RC, Cummins KW (1974) Leaf processing in a woodland stream. Freshw Biol 4:343–368. https://doi.org/10.1111/j.1365-2427.1974.tb00103.x
Pinna M, Fonnesu A, Sangiorgio F, Basset A (2004) Influence of summer drought on spatial patterns of resource availability and detritus processing in Mediterranean stream sub-basins (Sardinia; Iltaly). Int Rev Hydrobiol 89(5–6):484–499. https://doi.org/10.1002/iroh.200410765
Quijano-Baron J, Carlier R, Rodriguez JF, Sandi SG, Saco PM, Wen L, Kuczera G (2022) And we thought the Millennium Drought was bad: assessing climate variability and change impacts on an Australian dryland wetland using an ecohydrologic emulator. Water Res 218:118487. https://doi.org/10.1016/j.watres.2022.118487
Ramsar Convention (1987) Convention on wetlands of international importance especially as waterfowl habitat. Ramsar; Iran; 2.2.1971 as amended by the Protocol of 3.12.1982 and the Amendments of 28.5.1987. Director; Office of International Standards and Legal Affairs. United Nations Educational; Scientific and Cultural Organization (UNESCO)
Sangiorgio F, Dragan S, Rosati I, Teodorof L, Staras M, Georgescu L et al (2008) Decomposition of reed swamp detritus in the Danube Delta: a case study of four eutrophic systems. Transit Water Bull 24(4):26–37. https://doi.org/10.1285/i1825229Xv2n4p26
Simon B, Kucserka T, Anda A (2018) Investigation of Salix alba and Populus tremula leaf litter decomposition in the area of Lake Balaton and Kis-Balaton Wetland. Acta Agrar Debr 74:159–162. https://doi.org/10.34101/actaagrar/74/1682
Simon B, Kucserka T, Anda A (2019) Investigation of the decomposition and leaching dynamics of Salix; Populus and mixed leaves in the area of Lake Balaton and Kis-Balaton Wetland. Acta Agrar Debr 1:119–124. https://doi.org/10.34101/actaagrar/1/2382
Soja G, Züger J, Knoflacher M, Kinner P, Soja AM (2013) Climate impacts on water balance of a shallow steppe lake in eastern Austria (Lake Neusiedl). J Hydrol 480:115–124. https://doi.org/10.1016/j.jhydrol.2012.12.013
Song Y-B, Zhou M-Y, Qin Y-L, Cornelissen JHC, Dong M (2021) Nutrient effects on aquatic litter decomposition of free-floating plants are species dependent. Glob Ecol Conserv 30:e01748. https://doi.org/10.1016/j.gecco.2021.e01748
Sorrell BK, Mendelssohn IA, McKee KL, Woods RA (2000) Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Ann Bot 86:675–685. https://doi.org/10.1006/anbo.2000.1173
Thevenot M, Dignac MF, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211. https://doi.org/10.1016/j.soilbio.2010.03.017
Van Straten G, Herodek S (1982) Estimation of algal growth parameters from vertical primary production profiles. Ecol Mod 15:287–311. https://doi.org/10.1016/0304-3800(82)90086-2
Van Dokkum HP, Slijkerman DME, Rossi L, Constantini ML (2002) Variation in the decomposition of Phragmites australis in a monomictic lake: the role of grammarids. Hydrobiol 482:69–77. https://doi.org/10.1023/A:1021295610780
Vári Á, Tóth VR (2017) Quantifying macrophyte colonisation strategies—a field experiment in a shallow lake (Lake Balaton, Hungary). Aquat Bot 136:56–60. https://doi.org/10.1016/j.aquabot.2016.09.006
Vörös L, Somogyi, B (2009) A Balaton algaegyüteseinek szerepe a tó vízminőségének alakításában (The role of Lake Balaton’s algae composition in water quality). In: Bíró P, Banczerowsky J (Eds.), A Balaton kutatásának 2008. évi eredményei (The results of the research of Lake Balaton in the year 2008). Hungarian Academy of Sciences, Budapest 7–16.
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A et al (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Sci 336:1438–1440. https://doi.org/10.1126/science.1219534
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Contributions
AA: conceptualization, validation, writing—original draft preparation, writing—review and editing. SzS: formal analysis, investigation, data curation. BS-G: methodology, data curation, software. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The manuscript has not been submitted to more than one journal for simultaneous consideration. The manuscript has not been published elsewhere previously.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anda, A., Simon, S. & Simon-Gáspár, B. Impacts of wintertime meteorological variables on decomposition of Phragmites australis and Solidago canadensis in the Balaton System. Theor Appl Climatol 151, 1963–1979 (2023). https://doi.org/10.1007/s00704-023-04370-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00704-023-04370-y