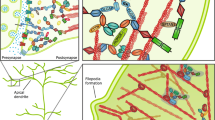
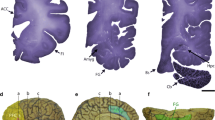
Abstract
Genetic and environmental factors increase autism spectrum disorder (ASD) incidence, and this has led to the generation of corresponding animal models, with some showing strong construct and face validity. This short review focuses on results we have recently obtained with environmental and genetic mouse models of ASD and that are the valproic acid, the poly I:C and the Shank 3 models. This has allowed us to provide a comparative description of these widely used animal models providing an interesting perspective as to the pros and cons of each one of them, in our experimental settings. In these papers, we focused on motor and gait disorders which are currently not included in the diagnosis criteria, but which may provide new insights to ASD pathophysiology potentially leading to innovative therapies for a disease that currently has none. In all these models, we reported behavioral, cellular and molecular alterations related to the cerebellum. Motor and gait deficits were observed to various degrees in animal models and, when strongly present, they were correlated to the severity of social deficits as well as to the number of cerebellar Purkinje cells. Additionally, we also reported that, like in humans, males are more severely affected than females in these ASD models. These findings, along with an increasing body of literature, open new hopes in the ASD field pointing to brain regions, such the cerebellum, that are at the crossroads between cognitive, social and motor deficits. Targeting these brain regions and their underlying pathways and synaptic connections may prove of significant benefits.
Similar content being viewed by others
References
Al Sagheer T, Haida O, Balbous A, Francheteau M, Matas M, Fernagut P-O, Jaber M (2018) Motor impairments correlate with social deficits and restricted neuronal loss in an environmental model of autism. Int J Neuropsychopharmacol 21(9):871–882
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, D.C
Arndt TL, Stodgell CJ, Rodier PM (2005) The teratology of autism. Int J Dev Neurosci 23(2–3):189–199
Asperger H (1944) Autistischen Psychopathen. Kindesalter Arch Psychiatr Nervenkr 117:76–136
Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40(12):1423–1430
Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P (1998) A clinicopathological study of autism. Brain J Neurol 121(Pt 5):889–905
Baron-Cohen S, Knickmeyer RC, Belmonte MK (2005) Sex differences in the brain: implications for explaining autism. Science 310(5749):819–823
Betancur C, Buxbaum JD (2013) SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism 4(1):17
Bey AL, Jiang YH (2014) Overview of mouse models of autism spectrum disorders. Curr Protoc Pharmacol 66(1):5–66
Boska P (2010) Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 24(6):881–897
Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y et al (2010) Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1(1):15
Burrows EL, Koyama L, May C, Hill-Yardin EL, Hannan AJ (2020) Environmental enrichment modulates affiliative and aggressive social behaviour in the neuroligin-3 R451C mouse model of autism spectrum disorder. Pharmacol Biochem Behav 195:172955
Chaste P, Leboyer M (2012) Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci 14(3):281–292
Christianson AL, Chesler N, Kromberg JG (1994) Fetal valproate syndrome: clinical and neuro- developmental features in two sibling pairs. Dev Med Child Neurol 36(4):361–369
Cook JL, Blakemore SJ, Press C (2013) Atypical basic movement kinematics in autism spectrum conditions. Brain J Neurol 136(Pt 9):2816–2824
Courchesne E (1997) Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 7(2):269–278
D’Mello AM, Stoodley CJ (2015) Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci 9:408
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G et al (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39(1):25–27
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A et al (2012) Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11(3):777–807
Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH (2010) Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord 40(10):1227–1240
Haida O, Al Sagheer T, Balbous A, Francheteau M, Matas M, Soria F, Fernagut P-O, Jaber M (2019) Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl Psychiatry 9(1):124
Hallmayer J (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095
Jeon SJ, Gonzales EL, Mabunga DFN, Valencia ST, Kim DG, Kim Y, Adil KJL, Shin D, Park D, Shin CY (2018) Sex-specific behavioral features of rodent models of autism spectrum disorder. Exp Neurobiol 27(5):321–343
Jiang Y-H, Ehlers MD (2013) Modeling autism by SHANK gene mutations in mice. Neuron 78(1):8–27
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17(1):45–59
Kanner L (1943) Autistic disturbances of affective contact. Acta Paedopsychiatr 35:100–136
Kaur M, Srinivasan SM, Bhat AN (2018) Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder. Res Dev Disabil 72:79–95
Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD (2019) Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 44(2):245–258
Kim JW, Park K, Kang RJ, Gonzales EL, Oh HA, Seung H, Ko MJ, Cheong JH, Chung C, Shin CY (2019) Gene-environment interaction counterbalances social impairment in mouse models of autism. Sci Rep 9(1):11490
Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, Liu S et al (2013) Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci 33(47):18448–18468
Kwon H-K, Choi GB, Huh JR (2022) Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol 43(3):230–244
Lacaria M, Corinne S, Gu W, Paylor R, Lupski JR (2012) Enriched rearing improves behavioral responses of an animal model for CNV-based autistic-like traits. Hum Mol Genet 21(14):3083–3096
Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, Giuliano F et al (2014) Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet 10(9):e1004580
Lin LZ, Zhan X-L, Jin C-Y, Liang J-H, Jing J, Dong G-H (2022) The epidemiological evidence linking exposure to ambient particulate matter with neurodevelopmental disorders: a systematic review and meta-analysis. Environ Res 209:112876
Loomes R, Hull L, Polmear W, Mandy L (2017) What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56(6):466–474
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520
Mabunga DFN, Gonzales ELT, Kim J-W, Kim KC, Shin CY (2015) Exploring the validity of valproic acid animal model of autism. Exp Neurobiol 24(4):285–300
Madore C, Leyrolle Q, Lacabanne C, Benmamar-Badel A, Joffre C, Nadjar A, Layé S (2016) Neuroinflammation in autism: plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural Plast 2016:3597209
Matas E, Maisterrena A, Thabault M, Balado E, Francheteau M, Balbous A, Galvan L, Jaber M (2021) Major motor and gait deficits with sexual dimorphism in a Shank3 mutant mouse model. Mol Autism 12(1):2
McCarthy MM (2016) Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci 18(4):361–372
Meyer U (2014) Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75(4):307–315
Miani A, Imbriani G, De Filippis G, De Giorgi D, Peccarisi L, Colangelo M, Pulimeno M et al (2021) Autism spectrum disorder and prenatal or early life exposure to pesticides: a short review. Int J Environ Res Public Health 18(20):10991
Mitchell EJ, Thomson DM, Openshaw RL, Bristow GC, Dawson N, Pratt JA, Morris BJ (2020) Drug-responsive autism phenotypes in the 16p11.2 deletion mouse model: a central role for gene-environment interactions. Sci Rep 10(1):12303
Monteiro P, Feng G (2017) SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci 18(3):147–157
Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ (2009) Decreased connectivity and cerebellar activity in autism during motor task performance. Brain J Neurol 132(Pt 9):2413–2425
Nicolini C, Fahnestock M (2018) The valproic acid-induced rodent model of autism. Exp Neurol 299:217–227
Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Macari S, Hepburn S, Rogers SJ (2008) Gross motor development, movement abnormalities, and early identification of autism. J Autism Dev Disord 38(4):644–656
Patterson PH (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204(2):313–321
Petroni V, Subashi E, Premoli M, Wöhr M, Crusio WE, Lemaire V, Pietropaolo S (2022) Autistic-like behavioral effects of prenatal stress in juvenile Fmr1 mice: the relevance of sex differences and gene-environment interactions. Sci Rep 12(1):7269
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276(39):36734–36741
Pierce K, Courchesne E (2001) Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry 49(8):655–664
Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD (2015) The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149:213–226
Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A (2013) Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci 110(13):5258–5262
Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J (1996) Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370(2):247–261
Rogers DC, Fisher EMC, Brown SDM, Peters J, Hunter AJ, Martin JE (1997) Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8(10):711–713
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30(1):80–89
Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, Gibson JM et al (2017) Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 20(12):1744–1751
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57(1):67–81
Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK (2006) A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord 36(6):779–793
Wang X, Kery R, Xiong Q (2018) Synaptopathology in autism spectrum disorders: complex effects of synaptic genes on neural circuits. Prog Neuropsychopharmacol Biol Psychiatry 84:398–415
Wilson HL, Wong ACC, Shaw SR, Tse W-Y, Stapleton GA, Phelan MC, Hu S, Marshall J, McDermid HE (2003) Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet 40(8):575–584
Zerbo O, Iosif A-M, Delwiche L, Walker C, Hertz-Picciotto I (2011) Month of conception and risk of autism. Epidemiology 22(4):469–475
Acknowledgements
The research leading to the results detailed in this review received funding from the Fondation pour la Recherche Médicale and the CPER-FEDER program. MJ is grateful for the Prebios animal facility staff for help and dedication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MJ declares that he has no financial or non-financial interests that are directly or indirectly related to the reported work in this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaber, M. Genetic and environmental mouse models of autism reproduce the spectrum of the disease. J Neural Transm 130, 425–432 (2023). https://doi.org/10.1007/s00702-022-02555-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02555-9