Abstract
Background
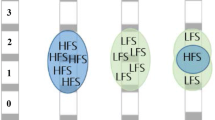
Subthalamic nucleus (STN) deep brain stimulation (DBS) alleviates severe motor fluctuations and dyskinesia in Parkinson’s disease, but may result in speech and gait disorders. Among the suspected or demonstrated causes of these adverse effects, we focused on the topography of contact balance (CB; individual, right and left relative dual positions), a scantly studied topic, analyzing the relationships between symmetric or non-symmetric settings, and the worsening of these signs.
Method
An observational monocentric study was conducted on a series of 92 patients after ethical approval. CB was specified by longitudinal and transversal positions and relation to the STN (CB sub-aspects) and totalized at the patient level (patient CB). CB was deemed symmetric when the two contacts were at the same locations relative to the STN. CB was deemed asymmetric when at least one sub-aspect differed in the patient CB. Baseline and 1-year characteristics were routinely collected: (i) general, namely, Unified Parkinson’s Disease Rating Scores (UPDRS), II, III motor and IV, daily levodopa equivalent doses, and Parkinson’s Disease Questionnaire of Quality of Life (PDQ39) scores; (ii) specific, namely scores for speech (II-5 and III-18) and axial signs (II-14, III-28, III-29, and III-30). Only significant correlations were considered (p < 0.05).
Results
Baseline characteristics were comparable (symmetric versus asymmetric). CB settings were related to deteriorations of speech and axial signs: communication PDQ39 and UPDRS speech and gait scores worsened exclusively with symmetric settings; the most influential CB sub-aspect was symmetric longitudinal position.
Conclusion
Our findings suggest that avoiding symmetric CB settings, whether by electrode positioning or shaping of electric fields, could reduce worsening of speech and gait.







Similar content being viewed by others
References
Aldridge D, Theodoros D, Angwin A, Vogel AP (2016) Speech outcomes in Parkinson’s disease after subthalamic nucleus deep brain stimulation: a systematic review. Parkinsonism Relat Disord 33:3–11
Alvarez L (2005) Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain 128(3):570–583
Antonini A, Moro E, Godeiro C, Reichmann H (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908
Askari A, Greif TR, Lam J, Maher AC, Persad CC, Patil PG (2022) Decline of verbal fluency with lateral superior frontal gyrus penetration in subthalamic nucleus deep brain stimulation for Parkinson disease. J Neurosurg 137(3):729–734
Barbe MT, Tonder L, Krack P et al (2020) Deep brain stimulation for freezing of gait in Parkinson’s disease with early motor complications. Mov Disord 35(1):82–90
Bender R, Lange S (2001) Adjusting for multiple testing—when and how? J Clin Epidemiol 54(4):343–349
Bohnen NI, Yarnall AJ, Weil RS, Moro E, Moehle MS, Borghammer P, Bedard M-A, Albin RL (2022) Cholinergic system changes in Parkinson’s disease: emerging therapeutic approaches. Lancet Neurol 21(4):381–392
Bove F, Mulas D, Cavallieri F et al (2021) Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology 97(3):1212–1246
Bruno S, Nikolov P, Hartmann CJ, Trenado C, Slotty PJ, Vesper J, Schnitzler A, Groiss SJ (2021) Directional deep brain stimulation of the thalamic ventral intermediate area for essential tremor increases therapeutic window. Neuromodulation 24(2):343–352
Butson CR, Cooper SE, Henderson JM, McIntyre CC (2007) Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34(2):661–670
Büttner C, Maack M, Janitzky K, Witt K (2019) The evolution of quality of life after subthalamic stimulation for Parkinson’s disease: a meta-analysis. Mov Disord Clin Pract 6(7):521–530
Cavallieri F, Budriesi C, Gessani A, Contardi S, Fioravanti V, Menozzi E, Pinto S, Moro E, Valzania F, Antonelli F (2021) Dopaminergic treatment effects on dysarthric speech: acoustic analysis in a cohort of patients with advanced Parkinson’s disease. Front Neurol 11(Article 616062):1–7
Chen CC, Brücke C, Kempf F, Kupsch A, Lu CS, Lee ST, Tisch S, Limousin P, Hariz M, Brown P (2006) Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr Biol 16(22):R952–R953
Chenausky K, MacAuslan J, Goldhor R (2011) Acoustic analysis of PD speech. Parkinsons Dis 2011:1–13
Chung SJ, Jeon SR, Kim SR, Sung YH, Lee MC (2006) Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur Neurol 56(2):127–132
Collomb-Clerc A, Welter M-L (2015) Effects of deep brain stimulation on balance and gait in patients with Parkinson’s disease: a systematic neurophysiological review. Neurophysiologie Clinique/Clin Neurophysiol 45(4–5):371–388
Coste J, Ouchchane L, Sarry L, Derost P, Durif F, Gabrillargues J, Hemm S, Lemaire JJ (2009) New electrophysiological mapping combined with MRI in Parkinsonian’s subthalamic region. Eur J Neurosci 29(8):1627–1633
De Bie RMA, Schuurman PR, Esselink RAJ, Bosch DA, Speelman JD (2002) Bilateral pallidotomy in Parkinson’s disease: a retrospective study. Mov Disord 17(3):533–538
de Chazeron I, Pereira B, Chereau-Boudet I, Durif F, Lemaire JJ, Brousse G, Ulla M, Derost P, Debilly B, Llorca PM (2016) Impact of localisation of deep brain stimulation electrodes on motor and neurobehavioural outcomes in Parkinson’s disease. J Neurol Neurosurg Psychiatr 87(7):758–766
de Roquemaurel A, Wirth T, Vijiaratnam N, Ferreira F, Zrinzo L, Akram H, Foltynie T, Limousin P (2021) Stimulation sweet spot in subthalamic deep brain stimulation – myth or reality? A critical review of literature. Stereotact Funct Neurosurg 99(5):425–442
Derost P-P, Ouchchane L, Morand D, Ulla M, Llorca P-M, Barget M, Debilly B, Lemaire J-J, Durif F (2007) Is DBS-STN appropriate to treat severe Parkinson disease in an elderly population? Neurology 68(17):1345–1355
Fabbri M, Guimarães I, Cardoso R et al (2017) Speech and voice response to a levodopa challenge in late-stage Parkinson’s disease. Front Neurol 8(Article 432):1–7
Feise RJ (2002) Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2(1):8
Fenoy AJ, McHenry MA, Schiess MC (2017) Speech changes induced by deep brain stimulation of the subthalamic nucleus in Parkinson disease: involvement of the dentatorubrothalamic tract. J Neurosurg 126(6):2017–2027
Florence G, Sameshima K, Fonoff ET, Hamani C (2016) Deep brain stimulation: more complex than the inhibition of cells and excitation of fibers. Neuroscientist 22(4):332–345
Fluchere F, Witjas T, Eusebio A, Bruder N, Giorgi R, Leveque M, Peragut J-C, Azulay J-P, Regis J (2014) Controlled general anaesthesia for subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 85(10):1167–1173
Follett KA, Weaver FM, Stern M et al (2010) Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362(22):2077–2091
Fox SH, Katzenschlager R, Lim S-Y, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266
Fraix V, Houeto J-L, Lagrange C et al (2006) Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatr 77(4):443–449
Garcia-Garcia D, Guridi J, Toledo JB, Alegre M, Obeso JA, Rodríguez-Oroz MC (2016) Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson’s disease: a new approach for active contact localization. J Neurosurg 1–12
Gonzalezballester M (2002) Estimation of the partial volume effect in MRI. Med Image Anal 6(4):389–405
Gonzalez-Escamilla G, Koirala N, Bange M, Glaser M, Pintea B, Dresel C, Deuschl G, Muthuraman M, Groppa S (2022) Deciphering the network effects of deep brain stimulation in Parkinson’s disease. Neurol Ther 11:265–282
Guiot G, Derome P, Trigo JC (1967) Intention tremor: the best indication for stereotaxic surgery. Presse Med 75(49):2513–2518
Harmsen IE, Elias GJB, Beyn ME, Boutet A, Pancholi A, Germann J, Mansouri A, Lozano CS, Lozano AM (2020) Clinical trials for deep brain stimulation: current state of affairs. Brain Stimul 13(2):378–385
Haynes WIA, Haber SN (2013) The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci 33(11):4804–4814
Hemm S, Coste J, Gabrillargues J et al (2009) Contact position analysis of deep brain stimulation electrodes on post-operative CT images. Acta Neurochir (Wien) 151(7):823–829 (discussion 829)
Holiga Š, Mueller K, Möller HE, Urgošík D, Růžička E, Schroeter ML, Jech R (2015) Resting-state functional magnetic resonance imaging of the subthalamic microlesion and stimulation effects in Parkinson’s disease: Indications of a principal role of the brainstem. Neuroimage Clin 9:264–274
Huang L-C, Chen L-G, Wu P-A, Pang C-Y, Lin S-Z, Tsai S-T, Chen S-Y (2022) Effect of deep brain stimulation on brain network and white matter integrity in Parkinson’s disease. CNS Neurosci Ther 28(1):92–104
Jiang J-L, Chen S-Y, Tsai S-T (2019) Quality of life in patients with Parkinson’s disease after subthalamic stimulation: an observational cohort study for outcome prediction. Ci Ji Yi Xue Za Zhi 31(2):107–112
Jourdain VA, Schechtmann G, Di Paolo T (2014) Subthalamotomy in the treatment of Parkinson’s disease: clinical aspects and mechanisms of action. J Neurosurg 120(1):140–151
Karachi C, Yelnik J, Tandé D, Tremblay L, Hirsch EC, François C (2005) The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord 20(2):172–180
Kim MJ, Chang KW, Park SH, Chang WS, Jung HH, Chang JW (2021) Stimulation-induced side effects of deep brain stimulation in the ventralis intermedius and posterior subthalamic area for essential tremor. Front Neurol 12:678592
Kim R, Kim H-J, Shin C, Park H, Kim A, Paek SH, Jeon B (2019) Long-term effect of subthalamic nucleus deep brain stimulation on freezing of gait in Parkinson’s disease. J Neurosurg 131(6):1797–1804
Kluin KJ, Mossner JM, Costello JT, Chou KL, Patil PG (2022) Motor speech effects in subthalamic deep brain stimulation for Parkinson’s disease. J Neurosurg 137(3):722–728
Knight EJ, Testini P, Min H-K et al (2015) Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clin Proc 90(6):773–785
Knowles T, Adams SG, Jog M (2021) Speech rate mediated vowel and stop voicing distinctiveness in Parkinson’s disease. J Speech Lang Hear Res 64(11):4096–4123
Krack P, Batir A, Van Blercom N et al (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349(20):1925–1934
Lemaire J-J, Coste J, Ouchchane L et al (2007) Brain mapping in stereotactic surgery: a brief overview from the probabilistic targeting to the patient-based anatomic mapping. Neuroimage 37(Suppl 1):S109-115
Lemaire J-J, De Salles A, Coll G, El Ouadih Y, Chaix R, Coste J, Durif F, Makris N, Kikinis R (2019) MRI atlas of the human deep brain. Front Neurol. https://doi.org/10.3389/fneur.2019.00851
Lemaire J-J, Pereira B, Derost P et al (2016) Subthalamus stimulation in Parkinson disease: Accounting for the bilaterality of contacts. Surg Neurol Int 7(Suppl 35):S837–S847
Lemaire J, Sakka L, Ouchchane L, Caire F, Gabrillargues J, Bonny J (2010) Anatomy of the human thalamus based on spontaneous contrast and microscopic voxels in high-field magnetic resonance imaging. Neurosurgery 66(3 Suppl Operative):161–172
Lezcano E, Gómez-Esteban JC, Tijero B et al (2016) Long-term impact on quality of life of subthalamic nucleus stimulation in Parkinson’s disease. J Neurol 263(5):895–905
Lhommée E, Wojtecki L, Czernecki V et al (2018) Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol 17(3):223–231
Limousin P, Foltynie T (2019) Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol 15(4):234–242
Lin Z, Zhang C, Li D, Sun B (2021) Lateralized effects of deep brain stimulation in Parkinson’s disease: evidence and controversies. npj Parkinsons Dis 7(1):64
Lizarraga KJ, Jagid JR, Luca CC (2016) Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation on gait kinematics in Parkinson’s disease: a randomized, blinded study. J Neurol 263(8):1652–1656
Lozano AM, Lipsman N, Bergman H et al (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15(3):148–160
Mueller K, Jech R, Růžička F et al (2018) Brain connectivity changes when comparing effects of subthalamic deep brain stimulation with levodopa treatment in Parkinson’s disease. Neuroimage Clin 19:1025–1035
Ni Z, Kim SJ, Phielipp N et al (2018) Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann Neurol 83(2):352–362
Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M (2008) Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord 23(Suppl 3):S548-559
Okun MS, Gallo BV, Mandybur G et al (2012) Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 11(2):140–149
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20(1):91–127
Pieruccini-Faria F, Ehgoetz Martens K, Silveira C, Jones J (2015) Side of basal ganglia degeneration influences freezing of gait in Parkinson’s disease. Behav Neurosci 129(2):214–218
Piña-Fuentes D, van Dijk JMC, van Zijl JC, Moes HR, van Laar T, Oterdoom DLM, Little S, Brown P, Beudel M (2020) Acute effects of adaptive deep brain stimulation in Parkinson’s disease. Brain Stimul 13(6):1507–1516
Prenger MTM, Madray R, Van Hedger K, Anello M, MacDonald PA (2020) Social symptoms of Parkinson’s disease. Parkinsons Dis 2020:1–10
Prent N, Potters WV, Boon LI, Caan MWA, de Bie RMA, van den Munckhof P, Schuurman PR, van Rootselaar A-F (2019) Distance to white matter tracts is associated with deep brain stimulation motor outcome in Parkinson’s disease. J Neurosurg 133(2):433–442
Rodriguez-Rojas R, Pineda-Pardo JA, Mañez-Miro J, Sanchez-Turel A, Martinez-Fernandez R, Del Alamo M, DeLong M, Obeso JA (2022) Functional topography of the human subthalamic nucleus: relevance for subthalamotomy in Parkinson’s disease. Mov Disord 37(2):279–290
Rossi M, Bruno V, Arena J, Cammarota Á, Merello M (2018) Challenges in PD patient management after DBS: a pragmatic review. Mov Disord Clin Pract 5(3):246–254
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46
Sandström L, Hägglund P, Johansson L, Blomstedt P, Karlsson F (2015) Speech intelligibility in Parkinson’s disease patients with zona incerta deep brain stimulation. Brain Behav 5(10):e00394
Sanger TD (2018) A computational model of deep-brain stimulation for acquired dystonia in children. Front Comput Neurosci 12:77
Schnitzler A, Mir P, Brodsky MA et al (2022) Directional deep brain stimulation for Parkinson’s disease: results of an international crossover study with randomized, double-blind primary endpoint. Neuromodulation: Technol Neural Interface 25(6):817–828
Schuepbach WMM, Rau J, Knudsen K et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–622
Shen L, Jiang C, Hubbard CS et al (2020) Subthalamic nucleus deep brain stimulation modulates 2 distinct neurocircuits. Ann Neurol 88(6):1178–1193
Stefani A, Cerroni R, Mazzone P, Liguori C, Di Giovanni G, Pierantozzi M, Galati S (2019) Mechanisms of action underlying the efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: central role of disease severity. Eur J Neurosci 49(6):805–816
Strotzer QD, Kohl Z, Anthofer JM, Faltermeier R, Schmidt NO, Torka E, Greenlee MW, Fellner C, Schlaier JR, Beer AL (2022) Structural connectivity patterns of side effects induced by subthalamic deep brain stimulation for Parkinson’s disease. Brain Connect 12:brain.2021.0051
Tanaka Y, Tsuboi T, Watanabe H et al (2020) Longitudinal speech change after subthalamic nucleus deep brain stimulation in Parkinson’s disease patients: a 2-year prospective study. J Parkinsons Dis 10(1):131–140
Thobois S, Ardouin C, Lhommee E et al (2010) Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain 133(4):1111–1127
Timmermann L, Jain R, Chen L et al (2015) Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol 14(7):693–701
Tir M, Devos D, Blond S et al (2007) Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of Parkinsonian patients. Neurosurgery 61(2):297–304 (discussion 304-305)
Tripoliti E, Limousin P, Foltynie T, Candelario J, Aviles-Olmos I, Hariz MI, Zrinzo L (2014) Predictive factors of speech intelligibility following subthalamic nucleus stimulation in consecutive patients with Parkinson’s disease: speech intelligibility after STN-DBS. Mov Disord 29(4):532–538
Tripoliti E, Zrinzo L, Martinez-Torres I et al (2011) Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology 76(1):80–86
van Hartevelt TJ, Cabral J, Deco G, Møller A, Green AL, Aziz TZ, Kringelbach ML (2014) Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. PLoS ONE 9(1):e86496
Vizcarra JA, Situ-Kcomt M, Artusi CA, Duker AP, Lopiano L, Okun MS, Espay AJ, Merola A (2019) Subthalamic deep brain stimulation and levodopa in Parkinson’s disease: a meta-analysis of combined effects. J Neurol 266(2):289–297
Vu TC, Nutt JG, Holford NHG (2012) Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol 74(2):267–283
Wang J-W, Zhang Y-Q, Zhang X-H, Wang Y-P, Li J-P, Li Y-J (2017) Deep brain stimulation of pedunculopontine nucleus for postural instability and gait disorder after Parkinson disease: a meta-analysis of individual patient data. World Neurosurg 102:72–78
Weintraub D, Duda JE, Carlson K, Luo P, Sagher O, Stern M, Follett KA, Reda D, Weaver FM (2013) Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson’s disease: results from a randomised, controlled trial. J Neurol Neurosurg Psychiatry 84(10):1113–1118
Welter M-L, Schüpbach M, Czernecki V et al (2014) Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology 82(15):1352–1361
Williams A, Gill S, Varma T et al (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9(6):581–591
Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T, Deuschl G (2013) Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain 136(Pt 7):2109–2119
Xie T, Kang UJ, Warnke P (2012) Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson’s disease. J Neurol Neurosurg Psychiatry 83(10):1015–1017
Yamamoto T, Uchiyama T, Higuchi Y, Asahina M, Hirano S, Yamanaka Y, Weibing L, Kuwabara S (2017) Long term follow-up on quality of life and its relationship to motor and cognitive functions in Parkinson’s disease after deep brain stimulation. J Neurol Sci 379:18–21
Zerroug A, Gabrillargues J, Coll G et al (2016) Personalized mapping of the deep brain with a white matter attenuated inversion recovery (WAIR) sequence at 1.5-tesla: experience based on a series of 156 patients. Neurochirurgie 62(4):183–9
Acknowledgements
We thank Dr. Vivien Mendes-Martins (Annecy, France), Dr. François Vassal (Saint-Etienne, France), Dr. François Caire (Limoges, France), Dr. Denys Fontaine (Nice, France), and Dr. Simone Hemm-Ode (Basel, Switzerland), for advice at the start of the study.
Author information
Authors and Affiliations
Contributions
Conceptualization, JJL, YEO, AM; methodology, BP, JJL, YEO; validation, YEO, ML, DM, JJL; formal analysis, JJL, YEO, BP; investigation, YEO, AM, ML, BD, PD, DM, FD, JJL; resources, YEO, BC, AS, JC, AM, BD, PD, JJL; data curation, YEO, DM, JC, FD; writing—original draft preparation, JJL, YEO, AM; writing-review and editing, YEO, AM, BP, ML, BC, JC, AS, RC, BD, PD, FD, JJL; visualization, JJL, YEO, BP; supervision, JJL; project administration, BP; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee: Ethical approval 2020/CE103, South-East VI; agreement approved March 3, 2021.
Informed consent statement
Informed consent was obtained from all the subjects involved in the study, or their representative.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
Although deterioration of speech and gait occurring in Parkinson disease (PD) patients over time is a well-known phenomenon; it occurs with or without deep brain stimulation (DBS) surgery and is frequently observed even in patients whose cardinal PD symptoms improve after surgery. Higher incidence of speech and gait worsening after bilateral surgical interventions has been known for decades, but the connection of this phenomenon to specific electrode contact locations, and especially their symmetry, remains unclear. The authors of this large cohort study make a strong argument that symmetricity of contact may be contributing, or directly causing, deterioration of axial issues and speech, but at the same time notice that this may occur with remarkable improvement in other PD symptoms. The question therefore remains if the intentional placement of DBS electrodes in asymmetric locations would eliminate negative effects on speech and gait while maintaining high efficacy for other motor PD manifestations, and whether quality of life in operated PD patients will be higher or lower with alternative electrode setups. It would probably be naïve to expect a panacea-like surgical intervention that would cure a complex neurodegenerative disorder with a simple delivery of electrical impulses, but it is definitely worth trying when it comes to optimization of our surgical approaches and technical nuances. I must admit that I never paid attention to intentional or unintentional symmetry of my implanted DBS electrodes; after reading this study, I would start doing it now.
Konstantin Slavin,
Chicago, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Ouadih, Y., Marques, A., Pereira, B. et al. Deep brain stimulation of the subthalamic nucleus in severe Parkinson’s disease: relationships between dual-contact topographic setting and 1-year worsening of speech and gait. Acta Neurochir 165, 3927–3941 (2023). https://doi.org/10.1007/s00701-023-05843-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05843-9