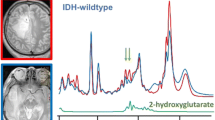
Abstract
Background
Increased extracellular glutamate is known to cause epileptic seizures in patients with glioblastoma (GBM). However, predicting whether the seizure will be refractory is difficult. The present study investigated whether evaluation of the levels of various metabolites, including glutamate, can predict the occurrence of refractory seizure in GBM by quantitative measurement of metabolite concentrations on magnetic resonance spectroscopy (MRS).
Methods
Forty patients were treated according to the same treatment protocol for primary GBM at Ehime University Hospital between April 2017 and July 2021. Of these patients, 23 underwent MRS to determine concentrations of metabolites, including glutamate, N-acetylaspartate, creatine, and lactate, in the tumor periphery by applying LC-Model. The concentration of each metabolite was expressed as a ratio to creatine concentration. Patients were divided into three groups: Type A, patients with no seizures; Type B, patients with seizures that disappeared after treatment; and Type C, patients with seizures that remained unrelieved or appeared after treatment (refractory seizures). Relationships between concentrations of metabolites and seizure types were investigated.
Results
In 23 GBMs, seizures were confirmed in 11 patients, including Type B in four and Type C in seven. Patients with epilepsy (Type B or C) showed significantly higher glutamate and N-acetylaspartate values than did non-epilepsy patients (Type A) (p < 0.05). No significant differences in glutamate or N-acetylaspartate levels were seen between Types B and C. Conversely, Type C showed significantly higher concentrations of lactate than did Type B (p = 0.001). Cutoff values of lactate-to-creatine, glutamate-to-creatine, and N-acetylaspartate-to-creatine ratios for refractory seizure were > 1.25, > 1.09, and > 0.88, respectively.
Conclusions
Extracellular concentrations of glutamate, N-acetylaspartate, and lactate in the tumor periphery were significantly elevated in patients with GBM with refractory seizures. Measurement of these metabolites on MRS may predict refractory epilepsy in such patients and could be an indicator for continuing the use of antiepileptic drugs.





Similar content being viewed by others
References
Bottomley PA (1987) Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 508:333–348
Buccoliero AM, Caporalini C, Scagnet M, Mussa F, Giordano F, Sardi I, Migliastro I, Moscardi S, Conti V, Barba C, Antonelli M, Gianno F, Rossi S, Diomedi-Camassei F, Gessi M, Donofrio V, Bertero L, Giangaspero F, Santi M, Aronica E, Genitori L, Guerrini R (2021) Angiocentric glioma-associated seizures: the possible role of EATT2, pyruvate carboxylase and glutamine synthetase. Seizure 86:152–154
Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H (2011) Glutamate release by primary brain tumors induces epileptic activity. Nat Med 2(17):1269–1274
Cardona AF, Rojas L, Wills B, Bernal L, Ruiz-Patiño A, Arrieta O, Hakim EJ, Hakim F, Mejía JA, Useche N, Bermúdez S, Carranza H, Vargas C, Otero J, Mayor LC, Ortíz LD, Franco S, Ortíz C, Gil-Gil M, Balaña C, Zatarain-Barrón ZL (2018) Efficacy and safety of levetiracetam vs. other antiepileptic drugs in Hispanic patients with glioblastoma. J Neurooncol 136(2):363–371
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, Pascual JM, Madden CJ, Mickey BE, Malloy CR, Bachoo RM, Maher EA (2012) 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 18:624–629
Dewan MC, Thompson RC, Kalkanis SN, Barker FG II, Hadjipanayis CG (2017) Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg 126:1772–1778
Dey S, Doddamani RS, Banerjee Dixit A, Tripathi M, Sharma MC, Chandra PS, Banerjee J (2021) Altered Spontaneous Glutamatergic and GABAergic Activity in the Peritumoral Cortex of Low-Grade Gliomas Presenting With History of Seizures. Front Neurosci 15:689769
French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, Trinka E, O’Brien TJ, Laurenza A, Patten A, Bibbiani F (2015) Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology 85:950–957
Garcia CG, Kahn SA, Geraldo LHM, Romano I, Domith I, Silva DCLE, Dos Santos AF, Ferreira MJ, Portugal CC, de Souza JM, Romão LF, Netto ADP, Lima FRS, Cossenza M (2018) Combination therapy with sulfasalazine and valproic acid promotes human glioblastoma cell death through imbalance of the intracellular oxidative response. Mol Neurobiol 55:6816–6833
Glavinas H, Krajcsi P, Cserepes J, Sarkadi B (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1:27–42
Huberfeld G, Vecht CJ (2016) Seizures and gliomas–towards a single therapeutic approach. Nat Rev Neurol 12:204–216
Inoue A, Ohnishi T, Kohno S, Ohue S, Nishikawa M, Suehiro S, Matsumoto S, Ozaki S, Fukushima M, Kurata M, Kitazawa R, Shigekawa S, Watanabe H, Kunieda T (2021) Met-PET uptake index for total tumor resection: identification of 11C-methionine uptake index as a goal for total tumor resection including infiltrating tumor cells in glioblastoma. Neurosurg Rev 44:587–597
Inoue A, Nishikawa M, Ohnishi T, Yano H, Kanemura Y, Ohtsuka Y, Ozaki S, Nakamura Y, Matsumoto S, Suehiro S, Yamashita D, Shigekawa S, Watanabe H, Kitazawa R, Tanaka J, Kunieda T (2021) Prediction of glioma stem like cell infiltration in the non-contrast-enhancing area by quantitative measurement of lactate on magnetic resonance spectroscopy in glioblastoma. World Neurosurg 153:e76–e95
Izumoto S, Miyauchi M, Tasaki T, Okuda T, Nakagawa N, Nakano N, Kato A, Fujita M (2018) Seizures and tumor progression in glioma patients with uncontrollable epilepsy treated with perampanel. Anticancer Res 38:4361–4366
Lo M, Wang YZ, Gout PW (2008) The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 215:593–602
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 131:803–820
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251
Nishikawa M, Inoue A, Ohnishi T, Kohno S, Ohue S, Matsumoto S, Suehiro S, Yamashita D, Ozaki S, Watanabe H, Yano H, Takahashi H, Kitazawa R, Tanaka J, Kunieda T (2018) Significance of glioma stem-like cells in the tumor periphery that express high levels of CD44 in tumor invasion, early progression, and poor prognosis in glioblastoma. Stem Cells Int 23:5387041
Nishikawa M, Inoue A, Ohnishi T, Yano H, Kanemura Y, Kohno S, Ohue S, Ozaki S, Matsumoto S, Suehiro S, Nakamura Y, Shigekawa S, Watanabe H, Kitazawa R, Tanaka J, Kunieda T (2021) CD44 expression in the tumor periphery predicts the responsiveness to bevacizumab in the treatment of recurrent glioblastoma. Cancer Med 10:2013–2025
Nishikawa M, Inoue A, Ohnishi T, Yano H, Ozaki S, Kanemura Y, Suehiro S, Ohtsuka Y, Kohno S, Ohue S, Shigekawa S, Watanabe H, Kitazawa R, Tanaka J, Kunieda T (2021) Hypoxia-induced phenotypic transition from highly invasive to less invasive tumors in glioma stem-like cells: significance of CD44 and osteopontin as therapeutic targets in glioblastoma. Transl Oncol 14(8):101137
Ogg RJ, Kingsley PB, Taylor JS (1994) WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B 104:1–10
Ohue S, Kohno S, Inoue A, Yamashita D, Harada H, Kumon Y, Kikuchi K, Miki H, Ohnishi T (2012) Accuracy of diffusion tensor magnetic resonance imaging-based tractography for surgery of gliomas near the pyramidal tract: a significant correlation between subcortical electrical stimulation and postoperative tractography. Neurosurgery 70:283–294
Ohue S, Kohno S, Inoue A, Yamashita D, Matsumoto S, Suehiro S, Kumon Y, Kikuchi K, Ohnishi T (2015) Surgical results of tumor resection using tractography-integrated navigation-guided fence-post catheter techniques and motor-evoked potentials for preservation of motor function in patients with glioblastomas near the pyramidal tracts. Neurosurg Rev 38:293–306
Okita Y, Nonaka M, Shofuda T, Kanematsu D, Yoshioka E, Kodama Y, Mano M, Nakajima S, Kanemura Y (2014) (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin Neurol Neurosurg 125:212–216
Pallud J, Le Van Quyen M, Bielle F, Pellegrino C, Varlet P, Cresto N, Baulac M, Duyckaerts C, Kourdougli N, Chazal G, Devaux B, Rivera C, Miles R, Capelle L, Huberfeld G (2014) Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med 6:24489
Piña-Garza JE, Rosenfeld W, Saeki K, Villanueva V, Yoshinaga H, Patten A, Williams B, Malhotra M (2020) Efficacy and safety of adjunctive perampanel in adolescent patients with epilepsy: Post hoc analysis of six randomized studies. Epilepsy Behav 104(Pt A):106876
Roh TH, Moon JH, Park HH, Kim EH, Hong CK, Kim SH, Kang SG, Chang JH (2020) Association between survival and levetiracetam use in glioblastoma patients treated with temozolomide chemoradiotherapy. Sci Rep 10(1):10783
Rösche J, Piek J, Hildebrandt G, Grossmann A, Kirschstein T, Benecke R (2015) Perampanel in the treatment of a patient with glioblastoma multiforme without IDH1 mutation and without MGMT promotor methylation. Fortschr Neurol Psychiatr 83:286–289
Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY (2015) Medical management of brain tumors and the sequelae of treatment. Neuro Oncology 17:488–504
Schiffer D, Annovazzi L, Casalone C, Corona C, Mellai M (2018) Glioblastoma: microenvironment and niche concept. Cancers (Basel) 11:5
Smith KL, Daniels JL, Arnoczky SP, Dodds JA, Cooper TG, Gottschalk A, Shaw DA (1994) Effect of joint position and ligament tension on the MR signal intensity of the cruciate ligaments of the knee. J Magn Reson Imaging 4(6):819–822
Smith SA, Levante TO, Meier BH, Ernst RR (1994) Computer simulations in magnetic resonance. An object oriented programming approach. J Magn Reson 106:75–105
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430
Vecht CJ, Kerkhof M, Duran-Pena A (2014) Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist 19:751–759
Villanueva V, Saiz-Diaz R, Toledo M, Piera A, Mauri JA, Rodriguez-Uranga JJ, López-González FJ, Gómez-Ibáñez A, Garcés M, González de la Aleja J, Rodríguez-Osorio X, Palao-Duarte S, Castillo A, Bonet M, Ruiz-Giménez J, Palau J, Arcediano A, Toledo M, Gago A (2016) NEOPLASM study: Real-life use of lacosamide in patients with brain tumor-related epilepsy. Epilepsy Behav 65:25–32
Ye ZC, Rothstein JD, Sontheimer H (1999) Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci 19(24):10767–10777
Yuen TI, Morokoff AP, Bjorksten A, D’Abaco G, Paradiso L, Finch S, Wong D, Reid CA, Powell KL, Drummond KJ, Rosenthal MA, Kaye AH, O’Brien TJ (2012) Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology 79:883–889
Acknowledgements
We are grateful to Taichi Furumochi and Yasuhiro Shiraishi from the Department of Neurology and to Satsuki Myoga from the Department of Pathology at Ehime University Hospital, Japan, for their help in obtaining pathological and radiological imaging findings. We also wish to thank Kohei Miura and Koji Maehara at GE Healthcare Japan for their advice regarding the technical background of MRS sequences and the postprocessing steps during the revision process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee for Clinical Research at Ehime University Hospital (approval No. 2110012) prior to initiation and was performed in accordance with the ethical standards as established in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent for participation in this study was obtained from all individual participants.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper. None of the authors has any financial interests to declare in relation to this work.
Open Access
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creative commons. org/ licenses/ by/4. 0/.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain Tumors
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 21 kb)
ESM 2
(PDF 64 kb)

ESM 3
Supplementary Fig. S1 Concentration of Creatine (Cr) in three types of epilepsy in glioblastoma (GBM). Type A: 4.43±0.72, Type B: 4.90±0.47, Type C: 3.51±1.24 (Type A vs Type B, p=0.638, Type A vs Type C, p=0.0985, Type B vs Type C, p=0.0534). There was no significant difference in the Cr concentration among patients showing three types of epileptic seizure. (PNG 299 kb)

ESM 4
Supplementary Fig. S2 Representative features of three types of epilepsy in GBM. (a) T1-weighted, gadolinium-enhanced imaging, (b) FLAIR, (c) MRS analysis using the LC-Model. Analysis of the concentrations of each metabolite (Glu, NAA, Lac, Cr) on MRS was performed by taking ROIs for target sites at the 11C-methionine uptake area with a TNR of 1.4 on methionine PET outside the gadolinium-contrast enhancing area on MRI (yellow square: target site). (PNG 2318 kb)

ESM 5
Supplementary Fig. S3 CD44 expression (presented by P/C ratio) in three types of tumor-related epilepsy reclassified by cutoff values of Glu/Cr, NAA/Cr, and Lac/Cr. Type A: Glu/Cr ≤1.09; NAA/Cr ≤0.88; and Lac/Cr ≤1.25. Type B: Glu/Cr >1.09; NAA/Cr >0.88; and Lac/Cr >1.25. Type C: Glu/Cr >1.09; NAA/Cr >0.88; and Lac/Cr >1.25. Values for the P/C ratio of CD44: Type A (n=6), 5.45±5.08; Type B (n=5), 1.06±0.95; and Type C (n=4), 11.86±4.91. Type A vs. Type B, p=0.2259; Type A vs. Type C, p=0.079; and Type B vs. Type C, p=0.0056. *p<0.01; ns, not significant.(PNG 436 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakamura, Y., Inoue, A., Nishikawa, M. et al. Quantitative measurement of peritumoral concentrations of glutamate, N-acetyl aspartate, and lactate on magnetic resonance spectroscopy predicts glioblastoma-related refractory epilepsy. Acta Neurochir 164, 3253–3266 (2022). https://doi.org/10.1007/s00701-022-05363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05363-y