Abstract
Background
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical diseases. In surgical management of CSDH, there is a lack of standardized guidelines concerning surgical techniques and a lack of consensus on which technique(s) are optimal. Neurosurgical centers have shown a wide variation in surgical techniques. The purpose of this study was to compare two different surgical techniques, one burr hole craniostomy with an active subgaleal drain (BHC) and minicraniotomy with a passive subdural drain (MC).
Methods
We conducted a multicenter retrospective cohort study at two neurosurgical centers in Sweden which included patients with unilateral CSDHs that received surgical treatment with either BHC or MC. The primary outcomes in comparison of the techniques were 30-day mortality, recurrence rate, and complications according to the Landriel Ibañez grading system for complications.
Results
A total of 1003 patients were included in this study. The BHC subgroup included 560 patients, and the MC subgroup included 443 patients. A 30-day mortality when comparing BHC (2.3%) and MC (2.7%) was similar (p = 0.701). Comparing recurrence rate for BHC (8.9%) and MC (10.8%) showed no significant difference (p = 0.336). We found that medical complications were significantly more common in the MC group (p = 0.001). Surgical complications (type IIb) was also associated with the MC group (n = 10, p = 0.003). Out of the 10 patients with type IIb complications in the MC group, 8 had postoperative acute subdural hematomas.
Conclusions
BHC was comparable to MC concerning 30-day mortality rate and recurrence rates. We did, however, find that MC was significantly associated with medical complications and serious surgical postoperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical diseases with a reported incidence of 8 to 14 per 100,000 person-years [14]. The incidence is expected to rise significantly, projecting the surgical evacuation of CSDH to become the most common neurosurgical procedure by 2030 [3]. The disease primarily affects the elderly population, and people with risk factors such as trauma, antithrombotic medication, anticoagulants, and alcohol abuse have been identified [10, 11, 14]. The increasing incidence already has a socio-economic impact on current healthcare systems, which together with recurrence rates estimated around 10–20% and a non-negligible surgical morbidity adds to the complexity of CSDH management [1, 4, 14]. Optimizing surgical treatment can help in lowering morbidity and recurrence rates, as well as minimizing the socio-economic impact.
The most common surgical technique is considered to be the burr hole craniostomy (BHC) where 1–2 burr holes are drilled. In the setting of 2 burr holes, they are drilled 5–8 cm apart to allow for effective irrigation of the hematoma [22]. An alternative to this is the minicraniotomy (MC) where 2–3 burr holes are drilled in close vicinity and joined together into a larger bone defect followed by irrigation. Previous data has shown that BHC has the best cure-to-complication ratio [29]. Furthermore, data on BHC have not been able to prove a difference in outcome when comparing 1 and 2 burr holes when performing BHC; there is however limited data on outcomes when performing BHC with one burr hole under local anesthesia [5, 25].
It is considered the standard of care today to insert a postoperative drain to allow for further drainage of hematoma as it has shown to decrease mortality and reduce the risk of recurrence of CSDH [23]. Initial evidence was shown for passive subdural drains, but lately evidence has shown less risk of recurrence when using an active subgaleal drain compared to the passive subdural drain [24].
The aim of this study was to assess burr hole craniostomy with active subgaleal drain in comparison to minicraniotomy with passive subdural drain with an emphasis on recurrence rate, mortality, and risk of complications.
Methods and materials
Study cohort
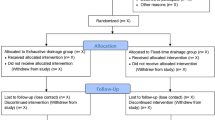
The following multicenter retrospective comparative cohort study took place at Scania University Hospital and Karolinska University Hospital. All patients over the age of 18 diagnosed with a surgically evacuated CSDH were eligible for the study. The study had the following exclusion criteria: bilateral CSDHs, cerebral shunts, simultaneous intracranial hemorrhages, and patients with permanent residency outside of Sweden. The patients included from Scania University Hospital were treated between 2012 and 2016, while the patients included from Karolinska University Hospital were treated between 2006 and 2014. During the time periods, the surgical techniques and management of CSDH were not altered at either center. A total of 1003 patients were included in this retrospective cohort study.
Surgical techniques
Patients were operated and managed with the following surgical techniques denoted as methods 1 and 2. Method 1 (i) was the standard surgical technique for evacuation of CSDH at Scania University Hospital. Method 2 (ii) was the standard surgical technique for evacuation of CSDH at Karolinska University Hospital. Surgeries were always to be conducted with local anesthesia unless special circumstances indicated the need of general anesthesia. In both centers, perioperative usage of antibiotics was administered prior to the initiation of surgery.
-
i.
Two or three burr holes combined into a minicraniotomy. The hematoma was evacuated with irrigation after which a passive subdural drain was placed in the subdural space for 24 h, while the patients were immobilized in bed. The passive drain did not have any active suction but rather functioned by natural pressure gradients.
-
ii.
Singular burr hole. The hematoma was evacuated with irrigation after which an active subgaleal drain was placed in the subgaleal space for 24 h, while the patients were immobilized in bed [24]. The active drain used active suction to drain the remaining fluids from the subdural space.
Variables
The data from this study was retrieved using the electric medical journal systems at both hospitals and the respective radiological image databases for both hospitals. Baseline characteristics for this study included age, gender (male/female), Charlson comorbidity index (CCI) variables (index which predicts mortality based on weighted comorbidities) [18], preoperative Glasgow Coma Scale (GCS) score, antithrombotic medications, use of vitamin K antagonist (VKA), and radiological densities of the hematomas on computed tomography scans graded as the largest portion of the hematoma being hypodense, isodense, hyperdense, or with mixed densities. All patients underwent a CT of the head preoperatively. No postoperative head CTs were routinely performed unless clinically indicated.
Radiological data was retrieved in the form of midline shift (mm) and largest hematoma diameter in the axial plane (mm). Surgical data was retrieved in the form of the type of anesthesia used (local with sedation or general anesthesia), drainage system duration, recurrence rate (defined as new evacuation of CSDH on the same side within 3 months of the initial evacuation), and mortality at 30 days and 1 year past surgical date. Complications were registered according to Landriel Ibañez (classification system for complications after neurosurgical procedures) [15]. Complications were defined as any deviation from the normal postoperative course occurring within 30 days of surgery. Reoperation was not registered as a complication in our study.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0, Armonk, NY, IBM Corporation. Descriptive statistics including measures such as frequency (n), percentages, mean, and median were employed to further describe subgroup characteristics. Univariate (independent samples t-test and Mann–Whitney), Chi-squared, Fisher’s exact test, and Cox regression were performed to assess the endpoints of this study. Alpha level of significance was defined as P-value < 0.05 in all analyses. Adjusted residuals > 2 was considered to indicate a significance level < 0.05.
Results
Baseline characteristics
A total of 1003 patients were included in our study cohort from two neurosurgical centers. A summary of the baseline characteristics can be found in Table 1. The majority of the study cohort consisted of males (68.2%) and were evenly distributed between the two treatment groups. The mean age of the study population at the time of diagnosis was 75 years. The two treatment groups had a similar distribution of underlying comorbidities defined as CCI > 1 point (33.9% vs 28.7%, p = 0.075 Mann–Whitney).
Antithrombotic treatment was evenly represented in both treatment groups. Anticoagulation in the form of VKA was more present in the MC group (20.5% vs 14.8%, p = 0.020 Mann–Whitney). The preoperative GCS scores were similar in both groups with a median of 15 and with most scores between 13 and 15 in both treatment groups (89.8% and 92.1%).
Data concerning surgical characteristics and outcome was also collected for the study cohort (Table 2). The mean hematoma diameter for the study cohort was 22.5 mm with comparable diameters in both treatment groups. The midline shift was however found to be significantly larger in the MC group (p = 0.013). Most patients were operated in local anesthesia with light sedation (97% in the BHC group vs 88.3% in the MC group). The drainage systems were inserted for similar durations (1.0 in the BHC group vs 1.2 days in the MC group).
Recurrence rates in the surgical treatment models
The recurrence rates for the entire study population were 9.8% (n = 98). Recurrence rate was 10.8% (n = 48) for patients operated with MC and 8.9% (n = 50) for patients operated with BHC. The odds ratio for reoperation after treatment with MC was 1.24 and non-significant as compared to surgery with BHC (p = 0.336, 95% CI 0.82–1.88).
Complication rates in the surgical treatment models
Complications were categorized by using the Landriel Ibañez system for complications. The study cohort had a total of 131 complications (13.1%) with 35 (6.3%) in patients who had undergone treatment with BHC. The remaining 96 complications (21.7%) were associated with MC (Table 2).
Complications were analyzed in regard to the two treatment groups with a statistically significant difference (p < 0.001). The analysis did however not give sufficient insight into the subgroups, and thus, post hoc testing was used yielding significant adjusted residual values (> 2) for Ibañez types Ia, Ib, and IIb (Table 3).
The complication types were compared between the treatment groups with exception for Ibañez IIIb where no cases were recorded. We identified complications categorized as Ibañez Ia, Ib, and IIb to have a significantly increased odds ratio of being present in the treatment group of MC (Table 3). As was previously indicated to us by the abnormally high adjusted residual values.
Detailed analysis of Ibañez IIb complications in the MC group revealed that out of the 10 reported IIb complications, 8 had postoperative acute subdural hematomas, 1 postoperative epidural hematoma, and 1 subdural empyema (Table 4).
30-day mortality rate in the surgical treatment models
Mortality was calculated from the time of surgery to the time of death. A 30-day and 1-year mortality was registered for all study patients. A 30-day mortality for the study cohort was found to be 2.5% (n = 25) with even distribution between the two treatment models (p = 0.696, Mann–Whitney). This was further tested in a survival function test. The hazard ratio for mortality at 30-day post-surgery with MC was 1.17 and non-significant as compared to BHC (p = 0.701, 95% CI 0.53–2.56, Cox regression) (Fig. 1).
Discussion
In this retrospective cohort study involving patients with unilateral CSDH undergoing surgery with two different techniques, BHC with active subgaleal drain or MC with passive subdural drain, we found no significant differences in outcome concerning mortality or recurrence. However, there was a significant difference in reported complications favoring BHC with active subgaleal drain compared to MC with passive subdural drain.
The recurrence rate in our study was in line with previously reported recurrence rates for CSDHs. When comparing our two surgical methods, we were not able to identify a significant difference in recurrence rate. Previous studies have highlighted the benefits of a MC due to better visualization and access to the hematoma and membranes [27]. The MC has been theorized to be better suited for recurrent CSDHs but also to prevent recurrence in the first place. There are however other studies with no major differences in outcomes such as recurrence when comparing MC to BHC [16, 20]. There is also data supporting the use of only one burr hole instead of two burr holes with similar results [5, 23, 28]. Regarding drainage techniques, initial studies proved the efficacy of subdural drains [23], whereas subsequent studies on active subgaleal drains seem to be of similar effectiveness without the risk of misplacement in the brain parenchyma. [9, 12, 13, 24, 26].
In our study, there was no difference in mortality between the groups. The 1-year mortality rate in our study cohort was 11.2%, which is in line with previous studies [19, 30]. This contrasts the 1-year mortality rate for the general Swedish population between 75 and 79 years of age being approximately 3% [6]. We thus confirm that CSDH seems to be a sentinel health event [8, 14, 17].
In our study, we found a higher rate of complications associated with MC. The rate of Ia and Ib complications (medical complications) in the MC group in our study was 16%, which is in line with previous studies [21, 27]. MC was also associated with significantly more type IIb complications. Previous studies have found an increased rate of serious complications and morbidity when operating with a craniotomy [7, 16]. However, it is important to note that those studies are referring to a more invasive technique compared to MC. Van der Weken et al. specifically studied MC but could not verify that the technique was associated with serious surgical complications [27].
The study is inherently limited by the retrospective study design. In this study, we chose to include unilateral hematomas, which are reflected in the relatively low recurrence rates. Bilateral CSDH’s are associated with an increased risk of recurrence [2, 31]. The study is limited by the dyssynchronous time periods between the two centers, even though we have no clear indications that this would affect the results regarding recurrence or complications. The strength of the study is the relatively large study population and a health care system where no patients with CSDH are treated in private clinics, reducing the risk of selection bias. It also enables us to register complications, especially severe complications requiring surgery, since no surgical complications will be handled outside the neurosurgical departments at our hospitals.
Conclusion
In this retrospective comparative cohort study, we found that the less invasive BHC technique holds at least equivalent effectiveness to MC. We also found that surgical evacuation by MC was significantly associated with medical complications as well as surgical complications, including postoperative acute subdural hematomas indicating a better safety profile when using the BHC technique.
Abbreviations
- CSDH:
-
Chronic subdural hematoma
- BHC:
-
One burr hole craniostomy with active subgaleal drain
- MC:
-
Minicraniotomy with passive subdural drain
- CCI:
-
Charlson comorbidity index
References
Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, Arjmand P, Baronia B, Reddy K, Murty N, Singh S (2014) Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg 259(3):449–457
Andersen-Ranberg NC, Poulsen FR, Bergholt B, Hundsholt T, Fugleholm K (2017) Bilateral chronic subdural hematoma: unilateral or bilateral drainage? J Neurosurg 126(6):1905–1911
Balser D, Farooq S, Mehmood T, Reyes M, Samadani U (2015) Actual and projected incidence rates for chronic subdural hematomas in United States veterans administration and civilian populations. J Neurosurg 123(5):1209–1215
Bartek J Jr, Sjåvik K, Kristiansson H, Ståhl F, Fornbe I, Förander P, Jakola AS (2017) Predictors of recurrence and complications after chronic subdural hematoma surgery: a population-based study. World Neurosurg 106:609–614
Belkhair S, Pickett G (2013) One versus double burr holes for treating chronic subdural hematoma meta-analysis. Can J Neurol Sci 40(1):56–60
Central Bureau of Statistics, Sweden. Statistics on death and population size in Sweden. https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/. Accessed Mar 8, 2021.
Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, Sussman E, Carpenter A Jr, Connolly ES (2012) The surgical management of chronic subdural hematoma. Neurosurg Rev 35(2):155–169
Dumont TM, Rughani AI, Goeckes T, Tranmer BI (2013) Chronic subdural hematoma: a sentinel health event. World Neurosurg 80(6):889–892
Glancz LJ, Poon MTC, Coulter IC, Hutchinson PJ, Kolias AG, Brennan PM, British Neurosurgical Trainee Research Collaborative (2019) Does drain position and duration influence outcomes in patients undergoing burr-hole evacuation of chronic subdural hematoma? Lessons from a UK Multicenter Prospective Cohort Study. Neurosurgery 85(4):486–493
Gulati S, Solheim O, Carlsen SM, Øie LR, Jensberg H, Gulati AM, Madsbu MA, Giannadakis C, Jakola AS, Salvesen Ø (2018) Risk of intracranial hemorrhage (RICH) in users of oral antithrombotic drugs: nationwide pharmacoepidemiological study. PLoS One 13(8):e0202575
Han MH, Ryu JI, Kim CH, Kim JM, Cheong JH, Yi HJ (2017) Predictive factors for recurrence and clinical outcomes in patients with chronic subdural hematoma. J Neurosurg 127(5):1117–1125
Häni L, Vulcu S, Branca M, Fung C, Z’Graggen WJ, Murek M, Raabe A, Beck J, Schucht P (2020) Subdural versus subgaleal drainage for chronic subdural hematomas: a post hoc analysis of the TOSCAN trial. J Neurosurg 133(4):1147–1155
Kamenova M, Wanderer S, Lipps P, Marbacher S, Mariani L, Soleman J (2020) When the drain hits the brain. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.02.166
Kolias AG, Chari A, Santarius T, Hutchinson PJ (2014) Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 10(10):570–578
Landriel Ibanez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, Tramontano R, Knezevich F, Carizzo A (2011) A new classification of complications in neurosurgery. World Neurosurg 75(5–6):709–715
Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC (2010) Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg 113(3):615–621
Miranda LB, Braxton E, Hobbs J, Quigley MR (2011) Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 114(1):72–76
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173(6):676–682
Rauhala M, Helén P, Seppä K, Huhtala H, Iverson GL, Niskakangas T, Öhman J, Luoto TM (2020) Long-term excess mortality after chronic subdural hematoma. Acta Neurochir (Wien) 162(6):1467–1478
Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC (2015) Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. https://doi.org/10.1371/journal.pone.0115085
Rohde V, Graf G, Hassler W (2002) Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev 25(1–2):89–94
Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ (2008) The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg 22(4):529–534
Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, Richards HK, Marcus H, Parker RA, Price SJ, Kirollos RW, Pickard JD, Hutchinson PJ (2009) Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet 374(9695):1067–1073
Sjåvik K, Bartek J Jr, Sagberg LM, Henriksen ML, Gulati S, Ståhl FL, Kristiansson H, Solheim O, Förander P, Jakola AS (2017) Assessment of drainage techniques for evacuation of chronic subdural hematoma: a consecutive population-based comparative cohort study. J Neurosurg. https://doi.org/10.3171/2016.12.JNS161713
Smith DM, Kishikova L, Norris MJ (2012) Surgical management of chronic subdural haematoma: one hole or two. Int J Surg 10(9):450–452
Soleman J, Lutz K, Schaedelin S, Kamenova M, Guzman R, Mariani L, Fandino J (2019) Subperiosteal vs subdural drain after burr-hole drainage of chronic subdural hematoma: a randomized clinical trial (cSDH-Drain-Trial). Neurosurgery 85(5):E825–E834
Van Der Veken J, Duerinck J, Buyl R, Van Rompaey K, Herregodts P, D’Haens J (2014) Mini-craniotomy as the primary surgical intervention for the treatment of chronic subdural hematoma–a retrospective analysis. Acta Neurochir (Wien) 156(5):981–987
Wan Y, Xie D, Xue Z, Xie J, Song Z, Wang Y, Yang S (2019) Single versus double burr hole craniostomy in surgical treatment of chronic subdural hematoma: a meta-analysis. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.07.097
Weigel R, Schmiedek P, Krauss JK (2003) Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry 74(7):937–943
Zolfaghari S, Ståhl N, Nittby Redebrandt H (2018) Does time from diagnostic CT until surgical evacuation affect outcome in patients with chronic subdural hematoma? Acta Neurochir (Wien) 160(9):1703–1709
Zolfaghari S, Bartek J Jr, Djärf F, Wong S, Strom I, Ståhl N, Jakola AS, Nittby Redebrandt H (2021) Risk factors for need of reoperation in bilateral chronic subdural hematomas. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-021-04811-5
Acknowledgements
We are grateful to Suhayb Ehsaan Ilaahi for language editing.
Funding
Open access funding provided by Lund University. This research was supported by Region Skåne ALF Fund [2019-YF0009].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The following study has been approved by the regional ethics committee on the respective study sites with ethical permit numbers EPN 2017/247 and EPN 2013/591–31/1.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The following paper has been submitted for presentation as an abstract poster at the EANS congress 2021 in Hamburg
This article is part of the Topical Collection on Brain trauma.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zolfaghari, S., Bartek, J., Strom, I. et al. Burr hole craniostomy versus minicraniotomy in chronic subdural hematoma: a comparative cohort study. Acta Neurochir 163, 3217–3223 (2021). https://doi.org/10.1007/s00701-021-04902-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04902-3