Abstract
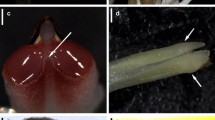
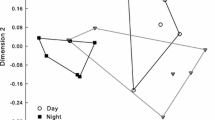
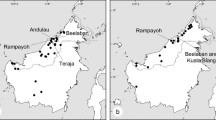
Members of Oncidiinae are widely known for their interactions with oil-collecting bees that explore lipophilic secretions on flowers. They may also be pollinated through food deception and the offering of nectar. Although data on breeding systems are available for many Oncidiinae orchids, little is known about the reproductive strategies in Rodriguezia, a neotropical genus of ca. 55 species. In this paper, we explore the reproductive biology of two species of Rodriguezia with distinctive morphologies: R. decora and R. lanceolata. Floral features, spectral reflectance, pollinators and pollination mechanisms, and breeding systems were studied. Both species are scentless and produce nectar as a reward. Floral nectar is secreted by a gland at the base of the labellum and stored into the sepaline spur. Rodriguezia decora reflects mainly in the blue and red regions of the light spectrum, while R. lanceolata reflects in the red region. Rodriguezia decora is exclusively visited and pollinated by butterflies, while Trochilidae hummingbirds are the pollinators of R. lanceolata. Pollinaria attach to the upper third of the proboscis of butterflies (R. decora), and to the bill of hummingbirds (R. lanceolata), during the collection of nectar from the spur. Both Rodriguezia species are self-sterile. Flower features and floral reflectance support the occurrence of psychophily in R. decora and ornithophily in R. lanceolata.




Similar content being viewed by others
References
Abdala-Roberts L, Parra-Tabla V, Navarro J (2007) Is floral longevity influenced by reproductive costs and pollination success in Cohniella ascendens (Orchidaceae)? Ann Bot (Oxford) 100:1367–1371. https://doi.org/10.1093/aob/mcm219
Aguiar JMRBV, Pansarin ER (2013) Does Oeceoclades maculata (Orchidaceae) reabsorbs nectar? Eur J Environm Sci 3:113–118
Aguiar JMRBV, Pansarin LM, Ackerman JD, Pansarin ER (2012) Biotic versus abiotic pollination in Oeceoclades maculata (Lindl.) Lindl. (Orchidaceae). Pl Spec Biol 27:86–95. https://doi.org/10.1111/j.1442-1984.2011.00330.x
Almeida AS, Vieira ICG (2010) Centro de Endemismo Belém: status da vegetação remanescente e desafios para a conservação da biodiversidade e restauração ecológica. Revista Estudos Univ 36:95–111
Bergamo PJ, Rech AR, Brito VLG, Sazima M (2016) Flower colour and visitation rates of Costus arabicus support the “bee-avoidance” hypothesis for red-reflecting hummingbird-pollinated flowers. Funct Ecol 30:710–720. https://doi.org/10.1111/1365-2435.12537
Carvalho R, Machado IC (2006) Rodriguezia bahiensis Rchb.f.: biologia floral, polinizadores e primeiro registro de polinização por moscas Acroceridae em Orchidaceae. Brazil J Bot 29:461–470. https://doi.org/10.1590/S0100-84042006000300013
Charanasri U, Kamemoto H (1977) Self-incompatibility in the Oncidium Alliance. Hawaii Orchid J 6:12–15
Chase MW, Williams NH, Faria AD, Neubig KM, Amaral MCE, Whitten WM (2009) Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): an expanded concept of Gomesa and a new genus Nohawilliamsia. Ann Bot (Oxford) 109:1–16. https://doi.org/10.1093/aob/mcp067
Cronk QCB, Ojeda I (2008) Bird-pollinated flowers in an evolutionary and molecular context. J Exp Bot 59:715–727. https://doi.org/10.1093/jxb/ern009
Cruden RW, Herman-Parker SM (1979) Butterfly pollination of Caesalpinia pulcherrima, with observations on the psychophilous syndrome. J Ecol 67:155–168. https://doi.org/10.2307/2259342
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, Oxford
Eguchi E, Watanabe K, Hariyama T, Yamamoto K (1982) A comparison of electrophysiologocally determined spectral responses in 35 species of Lepidoptera. J Insect Physiol 28:675–682
Faegri K, van der Pijl L (1979) The principles of pollination ecology. Pergamon Press, Oxford
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Fleming TH, Kress WJ (2013) The ornaments of life: coevolution and conservation in the tropics. University of Chicago Press, Chicago
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Co, New York
Kearns CA, Inouye D (1993) Techniques for pollinations biologists. University Press of Colorado, Niwot
Köppen W (1948) Climatologia: com um estúdio de los climas de la tierra. Fondo de Cultura Econômica, México
Leitão CAE, Cortelazzo AL (2008) Structural and histochemical characterization of the colleters of Rodriguezia venusta (Orchidaceae). Austral J Bot 56:161–165. https://doi.org/10.1071/BT07114
Leitão CAE, Dolder MAH, Cortelazzo AL (2014) Anatomy and histochemistry of the nectaries of Rodriguezia venusta (Lindl.). Rchb. f. (Orchidaceae). Flora 209:233–243. https://doi.org/10.1016/j.flora.2014.03.002
Leitão-Filho HF (1992) A flora arbórea da Serra do Japi. In: Morellato LPC (ed) História natural da Serra do Japi. Editora da Unicamp/Fapesp, Campinas, pp 40–62
Micheneau C, Fournel J, Warren BH, Hugel S, Gauvin-Bialecki A, Pailler T, Strasberg D, Chase MW (2010) Orthoptera, a new order of pollinator. Ann Bot (Oxford) 105:355–364. https://doi.org/10.1093/aob/mcp299
Montalvo AM, Ackerman JD (1987) Limitations to fruit production in Ionopsis utricularioides (Orchidaceae). Biotropica 19:24–31
Nunes CEP, Castro MM, Galetto L, Sazima M (2013) Anatomy of the floral nectary of ornithophilous Elleanthus brasiliensis (Orchidaceae: Sobralieae). Bot J Linn Soc 171:764–772. https://doi.org/10.1111/boj.12024
Ospina-Calderón NH, Duque-Buitrago CA, Tremblay RL, Tupac-Otero J (2015) Pollination ecology of Rodriguezia granadensis (Orchidaceae). Lankesteriana 15:129–139
Pansarin ER (2003) Biologia reprodutiva e polinização em Epidendrum paniculatum Ruiz & Pavón (Orchidaceae). Revista Brasil Bot 26:203–211. https://doi.org/10.1590/S0100-84042003000200008
Pansarin ER, Amaral MCE (2008) Reproductive biology and pollination mechanisms of Epidendrum secundum (Orchidaceae). Floral variation: a consequence of natural hybridization? Pl Biol 10:211–219. https://doi.org/10.1111/j.1438-8677.2007.00025.x
Pansarin ER, Ferreira AWC (2015) Butterfly pollination in Pteroglossa (Orchidaceae, Orchidoideae): a comparative study on the reproductive biology of two species of a Neotropical genus of Spiranthinae. J Pl Res 128:459–468. https://doi.org/10.1007/s10265-015-0707-x
Pansarin ER, Pansarin LM (2010) The family Orchidaceae in the Serra do Japi, State of São Paulo, Brazil. Springer, Wien. https://doi.org/10.1007/978-3-211-99755-0
Pansarin ER, Pansarin LM (2011) Reproductive biology of Trichocentrum pumilum: an orchid pollinated by oil-collecting bees. Pl Biol 13:576–581. https://doi.org/10.1111/j.1438-8677.2010.00420.x
Pansarin ER, Pansarin LM (2014) Floral biology of two Vanilloideae (Orchidaceae) primarily adapted to pollination by euglossine bees. Pl Biol 16:1104–1113. https://doi.org/10.1111/plb.12160
Pansarin ER, Bittrich V, Amaral MCE (2006) At daybreak—reproductive biology and isolating mechanisms in Cirrhaea dependens (Orchidaceae). Pl Biol 8:494–502. https://doi.org/10.1055/s-2006-923800
Pansarin ER, Pansarin LM, Santos IA (2015) Floral features, pollination biology, and breeding system of Comparettia coccinea (Orchidaceae: Oncidiinae). Flora 207:57–63. https://doi.org/10.1016/j.flora.2015.09.008
Pansarin ER, Santos IA, Pansarin LM (2017) Comparative reproductive biology and pollinator specificity among sympatric Gomesa (Orchidaceae: Oncidiinae). Pl Biol 19:147–155. https://doi.org/10.1111/plb.12525
Parra-Tabla V, Magaña-Rueda S (2000) Effects of deforestation on the reproductive ecology of Oncidium ascendens (Orchidaceae). Tropical bees: management and diversity. In: Munn, P. (ed), Proceedings of the VI international conference on tropical bees. San José de Costa Rica. IBRA, Cardiff, UK
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2009) Genera Orchidacearum 5: Epidendroideae (part two). Oxford University Press, New York
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Timber Press, Portland
Purvis MJ, Collier DC, Walls D (1964) Laboratory techniques in botany. Butterwoths, London
Rodríguez-Gironés MA, Santamaría L (2004) Why are so many birdflowers red? PLoS Biol 2:e350. https://doi.org/10.1371/journal.pbio.0020350
Rodríguez-Robles JA, Meléndez EJ, Ackerman JD (1992) Effects of display size, flowering phenology, and nectar availability on effective visitation frequency in Comparettia falcata (Orchidaceae). Amer J Bot 79:1009–1017
Ruschi A (1989) Aves do Brasil, Beija-flores. Vol. IV. Editora Expressão e Cultura, Rio de Janeiro
Sakai WS (1973) Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technol 43:247–249
Shrestha M, Dyer AG, Boyd-Gerny S, Wong BBM, Burd M (2013) Shades of red: bird-pollinated flowers target the specific colour discrimination abilities of avian vision. New Phytol 198:30–310. https://doi.org/10.1111/nph.12135
Simpson BB, Neff JL (1981) Floral rewards: alternatives to pollen and nectar. Ann Missouri Bot Gard 68:301–322. https://doi.org/10.2307/2398800
Singer RB, Koehler S (2003) Notes on the pollination of Notylia nemorosa (Orchidaceae): Do pollinators necessarily promote cross pollination? J Pl Res 116:19–25. https://doi.org/10.1007/s10265-002-0064-4
Singer RB, Sazima M (2000) The pollination of Stenorrhynchos lanceolatus (Aublet) L. C. Rich. (Orchidaceae: Spiranthinae) by hummingbirds in southeastern Brazil. Pl Syst Evol 223:221–227
Stiles FG (1981) Geographical aspects of bird- flower coevolution with particular reference to Central America. Ann Missouri Bot Gard 68:323–351
Stiles FG, Freeman CE (1993) Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25:191–205
Telles FJ, Kelber M, Rodríguez-Gironés MA (2016) Wavelength discrimination in the hummingbird hawkmoth Macroglossum stellatarum. J Exp Biol 219:553–560. https://doi.org/10.1242/jeb.130484
Torretta JP, Gomiz NE, Aliscioni SS, Bello ME (2011) Biología reproductiva de Gomesa bifolia (Orchidaceae, Cymbidieae, Oncidiinae). Darwiniana 49:16–24
Vale A, Navarro L, Rojas D, Álvarez JC (2011) Breeding system and pollination by mimicry of the orchid Tolumnia guibertiana in Western Cuba. Pl Spec Biol 26:163–173. https://doi.org/10.1111/j.1442-1984.2011.00322.x
van der Cingel NA (2001) An atlas of orchid pollination. America, Africa, Asia and Australia. Balkema Publishers, Rottherdam
van der Pijl L, Dodson CH (1966) Orchid flowers: their pollination and evolution. University of Miami, Coral Gables
Acknowledgements
The authors thank André V.L. Freitas (UNICAMP) for the identification of the butterflies. A.W.C.F. thanks FAPEMA for funding this research (grant 0430/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Ricarda Riina.
Rights and permissions
About this article
Cite this article
Pansarin, E.R., Bergamo, P.J., Ferraz, L.J.C. et al. Comparative reproductive biology reveals two distinct pollination strategies in Neotropical twig-epiphyte orchids. Plant Syst Evol 304, 793–806 (2018). https://doi.org/10.1007/s00606-018-1510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-018-1510-7