Abstract
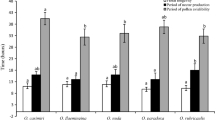
This paper reports on phenology, breeding system and dependence on pollinators of two sympatric Dichorisandra species. Dichorisandra incurva is a chamaephyte with continuous growth and precocious flowering, whereas D. hexandra is a geophyte with seasonal growth and delayed flowering. Flowering and fruiting occur mainly in the wet season; thus moisture can be important for both species. Besides moisture, photoperiod seems also to be related to the flowering only of D. incurva. Flowers of both species are poricidal, oligandrous, zygomorphic and buzz-pollinated by Apidae and/or Halictidae bees. Dichorisandra incurva is self-compatible, while D. hexandra shows a late-acting self-incompatible system. Moreover, herkogamy and poricidal anthers prevent spontaneous self-pollination in D. incurva. Therefore, medium- to large-sized Apidae are the most suitable pollinators as they make regular contact with anthers and stigmas and are further known to perform trapline foraging. This behaviour is particularly important for D. hexandra where Halictidae are considered pollen robbers, thus wasting pollen which, consequently, causes low reproductive efficacy in this species. Nevertheless, bee diversity is probably advantageous for the reproductive success of both Dichorisandra species. While the efficiency of each bee species is not known, further studies will show whether additional benefits are gained from the maintenance of these interactions.


Similar content being viewed by others
References
Alexander MP (1980) A versatile stain for pollen, fungi, yeast and bacteria. Stain Technol 55:13–18
Ando T, Nomura M, Tsukahara J, Watanabe H, Kokubun H, Tsukamoto T, Hashimoto G, Marchesi E, Kitching IJ (2001) Reproductive isolation in a native population of Petunia sensu Jussieu (Solanaceae). Ann Bot (Oxford) 88:403–413
Aona LYS, Amaral MCE (2009) Flora da Serra do Cipó, Minas Gerais: Commelinaceae. Bol Bot Univ São Paulo 27:253–258
Aona LYS; Pellegrini MOO (2014). Commelinaceae. In: Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB6924. Accessed 28 June 2014
Aona LYS, Faden RB, Amaral MCE (2011) Five new species of Dichorisandra J. C. Mikan (Commelinaceae) from Bahia State Brazil. Kew Bull 66:479–491
Aona-Pinheiro LYS, Amaral MCE (2012) Four new species of Dichorisandra J.C. Mikan (Commelinaceae) from Southeast Brazil. Phytotaxa 48:7–22
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell D, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Batalha MA, Martins FR (2004) Reproductive phenology of the Cerrado plant community in Emas National Park (central Brazil). Austral J Bot 52:149–161
Benavides AN, Wolf JHD, Duivenvoorden JF (2006) Recovery and succession of epiphytes in upper Amazonian fallows. J Trop Ecol 22:705–717
Boaventura YMS, Mathes LAF (1987) Aspectos da biologia da reprodução em plantas ornamentais cultivadas no estado de São Paulo: i – Dichorisandra thyrsiflora Mikan (Commelinaceae). Acta Bot Brasil 1:189–199
Buchmann SL (1983) Buzz pollination in Angiosperms. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold Inc., New York, pp 73–113
Charlesworth B, Charlesworth D (2000) Reproductive isolation: natural selection at work. Curr Biol 10:R68–R70
Coleman JR, Coleman MA (1982) Reproductive biology of an andromonoecious Solanum (S. palinacanthum Dunal). Biotropica 14:69–75
Cuevas J, Polito VS (2004) The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind-pollinated taxon. Ann Bot (Oxford) 93:547–553
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, Oxford
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite package: analysing ecological networks. R News 8:8–11
Faden RB (1983) Isolating mechanisms among five sympatric species of Aneilema R.Br. (Commelinaceae) in Kenya. Bothalia 14:997–1002
Faden RB, Hunt DR (1991) The classification of the Commelinaceae. Taxon 40:19–31
Frankie GW, Haber WA, Opler PA, Bawa KS (1983) Characteristics and organization of the large bee pollination system in the Costa Rican dry forest. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold Inc., New York, pp 411–447
Gardner M, Macnair M (2000) Factors affecting the co-existence of the serpentine endemic Mimulus nudatus Curran and its presumed progenitor, Mimulus guttatus Fischer ex DC. Biol J Linn Soc 69:443–459
Gotelli NJ, Entsminger GL (2000) EcoSiM: Null models software for ecology. Version 7 Acquired Intelligence Inc. & Kesey-Bear, Jericho VT. 05465. http://garyentsminger.com/ecosim/index.htm. Accessed 04 July 2013
Grossman GD (1986) Food resources partitioning in a rocky intertidal fish assemblage. J Zool 1:317–355
Hardy CR, Stevenson DW, Kiss HG (2000) Development of the gametophytes, flower, and floral vasculature in Dichorisandra thyrsiflora (Commelinaceae). Amer J Bot 87:1228–1239
Hopkins MJG, Hopkins HCF, Sothers CA (2000) Nocturnal pollination of Parkia velutina by Megalopta bees in Amazonia and its possible significance in the evolution of chiropterophily. J Trop Ecol 16:733–746
Hrycan WC, Davis AR (2005) Comparative structure and pollen production of the stamens and pollinator-deceptive staminodes of Commelina coelestis and C. dianthifolia (Commelinaceae). Ann Bot (Oxford) 95:1113–1130
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205
Johnson J, Omland K (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Kaul V, Koul AK (2008) Floral phenology in relation to pollination and reproductive output in Commelina caroliniana (Commelinaceae). Austral J Bot 56:59–66
Kaul V, Koul AK (2009) Sex expression and breeding strategy in Commelina benghalensis L. J Biosci 34:977–990
Kawai Y, Kudo G (2009) Effectiveness of buzz pollination in Pedicularis chamissonis: significance of multiple visits by bumblebees. Ecol Res 24:215–223
Kouonon LC, Jacquemart AL, Bi AIZ, Bertin P, Baudoin JP, Dje Y (2009) Reproductive biology of the andromonoecious Cucumis melo subsp. agrestis (Cucurbitaceae). Ann Bot 104:1129–1139
Kovach W (2004) Oriana Version 2.0 for Windows. Kovach Computing Services, Anglesey,Wales
Larson BMH, Barret SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Lloyd DG, Webb CJ, Dulberger R (1990) Heterostyly in species of Narcissus (Amaryllidaceae) and Hugonia (Linaceae) and other disputed cases. P1 Syst Evol 172:215–227
Maia DC (2006) Estudo taxonômico dos gêneros Commelina L. e Dichorisandra J.C. Mikan (Commelinaceae), no estado do Paraná, Brasil. Dissertation. Federal University of Paraná
Marques MCM, Roper JJ, Salvalaggio APB (2004) Phenological patterns among plant life-forms in a subtropical forest in southern Brazil. Pl Ecol 173:203–213
Martin FW (1959) Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol 34:125–128
Mikich SB, Silva SM (2001) Composição florística e fenologia das espécies zoocóricas de remanescentes de floresta estacional semidecidual no Centro-Oeste do Paraná Brasil. Acta Bot Brasil 15:89–113
Monasterio M, Sarmiento G (1976) Phenological strategies of plant species in the tropical savannah and the semi-deciduous forest of the Venezuelan Llanos. J Biogeogr 3:325–355
Morellato PC (1995) As estações do ano na floresta. In: Leitão Filho HF, Morellato LP (eds) Ecologia e preservação de uma floresta tropical urbana – reserva de Santa Genebra. Editora da Unicamp, Campinas. pp 37–41
Morellato LPC, Leitão Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28:180–191
Morellato LPC, Alberti LF, Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR (eds) Phenological Research: methods for environmental and climate change analysis. Springer, London, pp 339–359
Neal PR, Anderson GJ (2005) Are ‘mating systems’ ‘breeding systems’ of inconsistent and confusing terminology in plant reproductive biology? or is it the other way around? Pl Syst Evol 250:173–185
Newstrom LE, Frankie GW, Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest tress at La Selva, Costa Rica. Biotropica 26:41–159
Ohashi K, Thomson JD (2009) Trapline foraging by pollinators: its ontogeny, economics and possible consequences for plants. Ann Bot (Oxford) 103:1365–1378
Oliveira PE (2008) Fenologia e biologia reprodutiva das espécies de Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora, vol 1. EMBRAPA Cerrados, Brasília, pp 274–290
Owens SJ (1981) Self-incompatibility in the Commelinaceae. Ann Bot (Oxford) 47:567–581
Pianka ER (1973) The structure of lizard communities. Annual Rev Ecol Syst 4:53–74
R Development Core Team (2012) R: a language and environment for statistical computing, R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org Accessed 03 July 2013
Radford AE, Dickinson WC, Massey JR, Bell CR (1974) Vascular plant systematics. Harper & Row Publishers, New York
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annual Rev Ecol Syst 16:179–214
Rocha-Filho LC, Krug C, Silva CI, Garófalo CA (2012) Floral resources used by Euglossini bees (Hymenoptera: apidae) in coastal ecosystems of the Atlantic Forest. Psyche 2012:1–13
Sarmiento G, Monasterio M (1983) Life forms and phenology. In: Bouliere F (ed) Ecosystems of the world: tropical savannas. Elsevier Science, Amsterdan, pp 79–108
Seavey SR, Bawa K (1986) Late-acting self-incompatibility in Angiosperms. Bot Rev 52:195–219
Snow AA, Spira TP, Simpson R, Klips RA (1996) The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH (eds) Floral biology: studies on floral evolution in animal-pollinated plants. Chapman Hall, New York, pp 191–216
Solomon BP (1986) Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae). Amer J Bot 73:1215–1221
Steiner J, Zillikens A, Kamke R, Feja EP, Falkenberg DB (2010) Bees and melittophilous plants of secondary Atlantic forest habitats at Santa Catarina island, southern Brazil. Oecologia Austral 14:16–39
Ushimaru A, Itagaki T, Ishii HS (2003) Floral correlations in an andromonoecious species, Commelina communis (Commelinaceae). Pl Spec Biol 18:103–106
van Schaik CP, Terborgh JW, Wright SJ (1993) The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Rev Ecol Syst 24:353–377
Vogel S (1978) Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ (ed) The pollination of flowers by insects. Academic Press, London, pp 89–96
Wcislo WT, Arneson L, Roesch K, Gonzalez V, Smith A, Fernández H (2004) The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera: halictidae): an escape from competitors and enemies? Biol J Linn Soc 83:377–387
Whalen MD, Costich DE (1986) Andromonoecy in Solanum. In: D’Arcy WG (ed) Solanaceae. Columbia University Press, New York, Biology and Systematics, pp 284–302
Yang C, Gituru RW, Guo Y (2007) Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biol J Linn Soc 90:37–48
Zapata TR, Arroyo MTK (1978) Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10:221–230
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice-Hall, New Jersey
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support to MRS and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to MS. We are also grateful to anonymous reviewers for valuable comments, the Fundação José Pedro de Oliveira for permission to work at Santa Genebra Reserve, S.R. de M. Pedro, for bee identification; Luciano Paganucci Queiroz for pollen tube studies; Camila Aoki and Luis Fernando Alberti for phenological analysis; and Augusto Cesar de Aquino Ribas for the visiting bee species graph by R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sigrist, M.R., Sazima, M. Phenology, reproductive biology and diversity of buzzing bees of sympatric Dichorisandra species (Commelinaceae): breeding system and performance of pollinators. Plant Syst Evol 301, 1005–1015 (2015). https://doi.org/10.1007/s00606-014-1131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1131-8