Abstract
The species from Bulbophyllinae are generally regarded as fly-pollinated: myophilous or sapromyophilous. The section Cirrhopetalum is characterized by umbellate inflorescence and elongated lateral sepals. The aim of the floral anatomical investigation (micromorphology, histochemistry, ultrastructure) in Bulbophyllum wendlandianum (section Cirrhopetalum) was the detection of secretory activity. The appendages of dorsal sepal and petals function as osmophores. The exudation is transported inside vesicles via granulocrine secretion. The cuticle stretches and forms swellings on the entire cell surface. Such swellings of cuticle on the appendages of dorsal sepal and petals were not previously described in Bulbophyllum species. The nectary is located in the central groove on the adaxial lip surface. It comprises epidermal epithelial cells and few subepidermal layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bulbophyllum Lindl. (subtribe Bulbophyllinae Schltr.) is a large pantropical genus (c. 2400 spp., Sieder et al. 2007, 2010). The species belonging to B. section Cirrhopetalum occur mainly in South and East Asia and on the islands of The Malay Archipelago, also on Madagascar (e.g. Ridley 1885; Seidenfaden 1979; Vermeulen 1991, 2008; Seidenfaden and Wood 1992; Comber 1990, 2001; Augustine and Kumar 2001; Pearce and Cribb 2002; Rao 2010; Hosseini 2011; Ong et al. 2011a; Hosseini et al. 2012; Chowlu et al. 2013). The characteristic features of Cirrhopetalum species are umbellate inflorescence and elongated lateral sepals. The species from Bulbophyllinae are generally regarded as fly-pollinated, being a geographical vicariant of the subtribe Pleurothallidinae Lindl. (Pridgeon and Stern 1983; Azevedo et al. 2007; Kowalkowska 2009). Flowers regarded as fly-pollinated are described as myophilous or sapromyophilous. The first syndrome is characterized by simple, actinomorphic flowers, relatively small and bright dull coloured (green or yellowish). Nectar is produced in open shallow nectaries. The flower odour is slightly sweet or unpleasant for human. Whereas, in the second syndrome, the enticement to sapromyophilous flowers is frequently based on deception. The flies are attracted by the scents, colours and surfaces, which imitate fly’s natural food sources or their brood sites. The specialized devices, such as one-way hairs, translucent “windows” or motile lips, contribute to pollination accuracy and effectiveness. The motile elements of perianth, such as lips and filiform appendages or hairs, are often present (Christensen 1994). Such elements were described by Vogel (2001) as “flickering bodies” (from German: Flimmerkörper, FB) or vibratile bodies. They are noted in Orchidaceae, Asclepiadaeae, Aristolochiaceae and Sterculiaceae. Their structure may be diverse. Generally, they have a few millimetres, are very light and set in motion by the least air instability (even caused by wing beat of flying insects). FB are usually well exposed in flowers, also by contrasting colour. When surrounding is almost static and the minimal air currents set in motion flickering bodies, they are more visible and distinctive for insects. They look like imitating something in motion: conspecifics, prey, etc. The paddle- or banner-like or sometimes thread-like processes (appendages) are also called paleae. They are arisen on the petals and dorsal sepal, e.g. in Bulbophyllum ornatissimum, B. rotshildianum, B. wendlandianum (Ossian 1983). The flower colours are primarily dull, greenish to purple brown, often with spots. Nectar may or may not be produced in sapromyophilous flowers. The odour is generally strong and disagreeable for human. It is sometimes comparable with smell of fungi, rotten meat, or further forms of decaying protein (Christensen 1994). The odour, the main floral attractant in fly pollination (van der Pijl and Dodson 1969; Jones and Gray 1976; Proctor et al. 1996), is produced in scent glands (osmophores), morphologically distinguishable or not from other floral parts (Stern et al. 1987; Vogel 1990). Osmophores could be localized in structures called “antennae”, which are swollen apices of petals and/or sepals (e.g. in Bulbophyllum cerambyx J. J. Sm., Myoxanthus reymondii (H. Karst.) Luer, Restrepia antennifera Kunth). They can be also localized in prolonged apices of tepals, especially sepals (in Dracula Luer, Masdevallia Ruiz & Pav.) or even as glands on the fused sepals (in Scaphosepalum verrucosum (Rchb. f.) Pfitzer (=Scaphosepalum ochthodes (Rchb. f.) Pfitzer) (van der Cingel 1995). In flowers of B. ornatissimum, two different groups of osmophores were noted (Vogel 1990). A smell of cod-liver oil is produced by tail osmophores on the distal part of the lateral sepals, whereas a trimethylamine odour is emitted from lip. Vogel (1990) also hypothesized that each odour works differently: the odour from tail osmophores, far from the gynostemium, can be a long-distance attractant, while aminoid odour and nectar on the lip could play a role as short-distance attractants. In Bulbophyllum ipanemense Hoehne, B. involutum Borba, Semir & F. Barros and B. weddellii (Lindl.) Rchb.f. (Teixeira et al. 2004), the papillose osmophores are located on the lobes and on the adaxial surface of the lip callus. Furthermore, in these species, the cavity of the lip callus functions as a nectary. The nectary is formed by an epithelial layer and two or three adjacent layers. The accumulation of the occasionally emitted substances from osmophores on their surfaces is not observed because of the cytotoxic activity (Vogel 1990). Silva et al. (1999), after comparison between results of Bulbophyllum weddelii, B. involutum, B. ipanemense with B. gracillimum Rolfe (section Cirrhopetalum) and Dracula chestertonii (Rchb.f.) Luer (subtribe Pleurothallidinae), claimed that fly-pollinated orchids release similar floral scent composition relating to the compound classes: n-alkyloketones, n-alkyl-aldehydes, n-alkyl-alcohols, aromatic and some terpenes. Moreover, fly-pollinated flowers of Bulbophyllum from section Sestochilos Benth. & Hook. f. produce compounds (not nectar), such as zingerone, raspberry ketone, methyl eugenol and phenylopropanoid components. Such compounds are collected by Bactrocera fruit-fly-pollinators as semio- and allochemicals. Zingerone and methyl eugenol are collected by male fruit-flies and usually converted into chemical component(s) of sex pheromones. Additionally, they could be transformed into allomones to deter predators (Tan 2008). The second feature of fly-pollinated flowers, a motile lip, is broadly known in the subtribe Bulbophyllinae (e.g. Ridley 1890; van der Cingel 1995; Borba and Semir 1998; Vogel 2001; Teixeira et al. 2004; Kowalkowska 2009; Ong 2011a; Ong et al. 2011b) and Pleurothallidinae (e.g. Luer 1982, 1987; Vogel 2001; van der Cingel 1995; Kowalkowska 2009; Merino et al. 2010). The mechanism of lip movement (presenting some differences in pollination between species) is precisely described, e.g. in B. macranthum Lindl. from section Stenochilus (Ridley 1890), B. auratum (Lindl.) Rchb.f.—section Cirrhopetalum (Jongejan 1994), B. psittacoides—section Cirrhopetalum (van der Cingel 1995), in B. patens—section Sestochilus Benth & Hook. f. (Tan and Nishida 2000; Ong 2011a), in B. baileyi—section Stenochilus J. J. Sm. (Tan and Nishida 2007), in B. praetervisum J. J. Sm. (Ong 2011b), in three species from section Racemosae (Ong and Tan 2012), and also observed in Pleurothallis luteola Lindl. (Singer and Cocucci 1999).
The flowers of species belonging to Bulbophyllum section Cirrhopetalum are arranged in an umbel. Knerr (1981) interpreted the floral arrangement in Bulbophyllum makoyanum (Rchb.f.) Ridl. as mimicking other nectar-offering radial flowers, which is still not proved by coexistence of the orchids with any native representatives of, e.g. Asteraceae in the same habitat. In flowers of B. ornatissimum, two different groups of osmophores were noted (Vogel 1990). A smell of cod-liver oil is produced by tail osmophores on the distal part of the lateral sepals, whereas a trimethylamine odour is emitted from lip. Vogel (1990) also hypothesized that each odour works differently: the odour from tail osmophores, far from the gynostemium, can be a long-distance attractant, while aminoid odour and nectar on the lip could play a role as short-distance attractants.
The aim of the floral anatomical investigation (micromorphology, histochemistry, ultrastructure) was the detection of secretory activity.
Materials and methods
Samples of Bulbophyllum wendlandianum (Fig. 1a, b) were collected from flowers at anthesis in December 2011 (voucher number O/80-664) and April 2012 (voucher number O/115-90) in Botanischer Garten der Universität Wien (WU). Fresh flowers were observed under a Nikon SMZ1500 stereomicroscope. Plant material was fixed in 2.5 % glutaraldehyde (GA) in 0.05 M cacodylate buffer (pH = 7.0). The material for LM was rinsed with cacodylate buffer and then dehydrated. Whole dehydrated material was embedded in epoxy resin (Spurr 1969) and methylmethacrylate-based resin (Technovit 7100, Heraeus Kulzer GmbH). Sections were cut with glass knives (1–5 μm thick) and mounted on glass slides. For LM, the material was stained with 0.05 % Toluidine Blue O (TBO) for 1 min at 60 °C on a hot plate (Feder and O’Brien 1968; Ruzin 1999). Aniline Blue Black (ABB, C.I. 20470) was used for detection of water-insoluble proteins (Jensen 1962). The PAS reaction was used to identify the presence of water-insoluble polysaccharides (Jensen 1962) and Sudan Black B for lipid localization (Bronner 1975). Starch grains were detected using polarized light. The preparations were examined and photographed with a Nikon Eclipse E 800 light microscope and a Nikon DS-5Mc camera using the Lucia Image software.
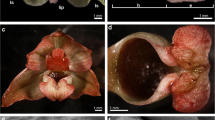
Bulbophyllum wendlandianum a plant (O/115-90 WU), phot shared by R. Hromniak; b single flower with indication of floral parts: the outer whorl built by dorsal sepal (ds) with branched appendages at the apex and two lateral sepals (ls) free at base, coherent above, attenuate at apices. The inner whorl built by two petals (pt) with branched appendages at the apices and lip. Gynostemium (g) with stelidia (st); c abaxial (outer) side of dorsal sepal with multicellular branched appendages at the apex and elongated appendages at the base (arrows) (LM); d groups of elevated cells indicated by white arrows (LM) present on the whole surface of abaxial surface of dorsal sepal; e magnification of d, groups of elevated cells (pink) (SEM); f cross-section of dorsal sepal with present in parenchyma three collateral vascular bundles (vb) and idioblasts with raphides (r) (PAS); g magnification of f, note the expanded raphides (arrow) pushing out the cells; h small lipid bodies visible at the base of dorsal sepal (SBB); i elongated appendages at the base (indicated by arrows at c) covered by strongly undulated cuticle, note large nucleus (n) and large drops (arrow) which are tannin-like materials
For scanning electron microscopy (SEM), after dehydration in an ethanol series the samples were dried by the critical point method using liquid CO2, coated with gold and observed in a Philips XL-30.
For transmission electron microscopy (TEM), the floral material was fixed in glutaraldehyde (2.5 % GA) in 0.05 M cacodylate buffer (pH 7.0). The material was post-fixed overnight in 1 % OsO4 in cacodylate buffer in a refrigerator and then rinsed in the buffer. After 1 h in 1 % uranyl acetate in distilled water, the material was dehydrated with acetone and embedded in Spurr’s resin. Ultrathin sections were cut on a Sorvall MT 2B ultramicrotome with a diamond knife and contrast stained with uranyl acetate and lead citrate. The sections were examined in a Philips CM 100 transmission electron microscope.
Samples were prepared in accordance with procedures described elsewhere (Kowalkowska et al. 2010, 2012; Kozieradzka-Kiszkurno and Płachno 2013).
Results
Micromorphology, histochemistry and ultrastructure
Dorsal sepal
Dorsal sepal (Fig. 1b, c) was pale greenish cream with dark red-coloured stripes, with multicellular branched appendages at apical part and elongated appendages at margins, shorter at the base. On the abaxial (outer) surface of dorsal sepal, the groups of elevated cells were visible (Fig. 1d, e). On the cross-section, it appeared that such elevations were caused by the raphide extension pushing out the cells (Fig. 1f, g). A large number of idioblasts with raphides were present in the parenchyma of dorsal sepal. On the cross-section, single layer of epidermal epithelial cells and three collateral vascular bundles in parenchyma were noticed (Fig. 1f). In cytoplasm, starch grains (Fig. 1f, g) were not noted. Small lipid bodies were visible at the base (Fig. 1h) and at the apex of the dorsal sepal. The cell walls of short appendages cells at the base of dorsal sepal (indicated by arrows at Fig. 1c) were covered by strongly undulated cuticle (Fig. 1i). The ellipsoidal hairs (Fig. 2b) and minute papille occur (Fig. 2c) on the abaxial (inner) surface at the apex (Fig. 2a). The undulated cuticular striations on epidermal cells were noted (Fig. 2b, c). The large drops visible in cells stained in TBO (Figs. 1i, 2e), after TEM studies, were detected as tannin-like materials (as in Fig. 5b). The protuberances observed in LM and SEM on the surface of multicellular branched appendages (Fig. 2d–f) were visible in TEM results as the swellings of cuticle proper (Figs. 2g, 3a–c). The cuticle was built by slightly reticulate cuticle layer (Fig. 3b, c) and extended in swellings cuticle proper (Figs. 2g, 3a–c). Swellings were noticed on the entire cell surface, sometimes at the points between adjoining epidermal cells (Fig. 3a). The meagre amount of lipoidal secretion was present on and between the cuticular swellings of appendages (Fig. 3b, c) and in larger quantity at the points between adjoining epidermal cells (Fig. 2f). In the appendages cells, parietal layer of cytoplasm contains plastids with plastoglobuli and intraplastidal membranes (Fig. 2g), abundant rough endoplasmic reticulum (rER) (Figs. 2g, 3a–c), mitochondria (Fig. 2g) and large central vacuole (Figs. 2g, 3a–c). The vesicles building into plasmalemma (Fig. 2g) and large invaginations of plasmalemma (Fig. 3c) were observed. Also tannin-like materials were noticed (not illustrated).
SEM, LM and TEM results of dorsal sepal illustrating a the abaxial (inner) surface at the apex built by ellipsoidal hairs (b) and minute papillae (c) (SEM); d protuberances—cuticle swellings on the surface of appendages visible at the apex of dorsal sepal (SEM); e cross-section of appendages with large drops of tannin-like material in cells (arrow) (TBO); f cross-section of appendages with the lipoidal secretion on the cuticular swellings and in larger quantity between epidermal cells (arrow) on abaxial (external) epidermis (ab) and adaxial (inner) epidermis (ad) (SBB); g cell of appendages with vesicles (ve) building into plasmalemma (pl) transporting substances through cell wall (cw), which accumulate below cuticle and then cuticle proper (cp) extending in swellings. Inside cell, parietal cytoplasm with abundant rough endoplasmic reticulum (rER), plastids (p) with plastoglobuli (white arrows) and internal tubules (white asterisks), mitochondrion (m) and large central vacuole (va) (TEM)
TEM images of the cells of appendages of dorsal sepal a large vacuole (va) with parietal layer of cytoplasm containing abundant rough endoplasmic reticulum (rER). The secretion exudates through cell wall (cw) and accumulates below cuticle consisted of reticulate cuticle layer (cl) and cuticle proper (cp), which forms swellings b swellings with meagre amount of exudate (black arrows) noted on the entire cell surface, sometimes at the points between adjoining epidermal cells c invaginations of plasmalemma (pl) (asterisks). LM and SEM images of lateral sepal d cross-section of the apex with tiny starch grains (black arrows) (LM, PAS); e magnification of d, tiny starch grains (white arrows) visible in polarized light (LM); SEM images illustrating the abaxial (outer) surface built by papillae with cuticular striations (f) and smooth cells or with slightly sculptured cuticle on the inner (adaxial) surface (g); h similarly as in dorsal sepal, the cells with fine lipid bodies and thin lipoidal layer on the epidermis (SBB)
Lateral sepals
Lateral sepals, forming with dorsal sepal the outer whorl, were coloured as dorsal sepal, free at base, coherent above, attenuate at purplish apices (Fig. 1a, b). The papillae with cuticular striations occurred on the abaxial (outer) surface of lateral sepal (Fig. 3f). On the adaxial (inner) surface, smooth cells or with slightly sculptured cuticle were observed (Fig. 3g). The cross-section of the apex revealed single layer of epidermis, one layer of subepidermal cells and parenchyma with intercellular spaces (Fig. 3d). Similarly as in dorsal sepal, fine lipid bodies in the cells and thin lipoidal layer on the epidermis were visible (Fig. 3h). Tiny starch grains (Fig. 3d; in polarized light—Fig. 3e), tannin-like materials and idioblasts with raphides were detected.
Petals
Pale cream with dark red stains petals (Figs. 1b, 4a), parts of inner whorl, had multicellular branched appendages at the apex and elongated appendages at the base, similarly to dorsal sepal from outer whorl. The adaxial (inner) surface was covered by flat cells (Fig. 4b) with undulated cuticle. At the apex, papillae and minute papillae with undulated cuticle were present (Fig. 4c). Sometimes the epidermal cells were visible as elevated groups (Fig. 4c), which was caused by pushing out them by extension of idioblasts with raphides. In the contraction between the apical part of petal and multicellular branched appendages, the cells were elongated with strong cuticular undulation (Fig. 4d). The outer (abaxial) surface was smooth. The multicellular branched appendages at the apex were built by elongated cells with strongly undulated cuticle with protuberances of different sizes (Fig. 4e, f). At the base of petal, protuberances were also noted on the elongated appendages. TEM results revealed that, as in dorsal sepal, the cuticle consisting of reticulate cuticle layer and cuticle proper, formed the swellings (visible as protuberances in SEM) up to 2 µm high (Fig. 5a, b). The swellings developed on the entire surface of cell wall, also at the connection points of adjoining epidermal epithelial cells. On their surface, at the end of cuticle reticulation (microchannels) (Fig. 5c–e), only a meagre amount of substances were sometimes noted (Fig. 5e), which were probably lipids (compare with Figs. 2f, 3b, c, h). In the petal cells, only fine lipid bodies were observed (not noted in the cells of appendages), with no proteins or starch grains. TEM studies (Fig. 5a–e) showed large central vacuole with tannin-like materials and parietal layer of cytoplasm with visible abundant rough endoplasmic reticulum (rER), oval-shaped and ameboidal plastids with plastoglobuli and internal tubules, mitochondria. The idioblasts with raphides were also noted.
LM and SEM images illustrating petal with a large multicellular branched appendages at the apex and elongated appendages at the base (LM); b the adaxial (inner) surface of petal covered by flat cells with undulated cuticle (SEM); c the cells at the apex of petal with groups of elevated cells (pink) (SEM); d elongated cells with strong cuticular undulation observed in the contraction between the apical part of petal and appendages (SEM). SEM images of multicellular branched appendages showing e elongated cells with protuberances—cuticle swellings; f note the different sizes of cuticle swellings
TEM images of multicellular branched appendages of petal a, b the cells with large central vacuole (va) with tannin-like materials (t) and parietal layer of cytoplasm with oval-shaped and ameboidal plastids (p). The cuticle (c) forming swellings up to 2 µm. The cuticle swellings develop on the entire surface of cell wall (cw), also at the connection points of adjoining epidermal cells; c large central vacuole (va) and parietal layer of cytoplasm with oval-shaped plastid (p), mitochondrion (m), abundant rough endoplasmic reticulum (rER); d, e cuticle consisting of reticulate cuticle layer (cl) and cuticle proper (cp). The exudation penetrates through cell wall (cw) and accumulates below reticulate cuticle layer (cl). The meagre amount of exudates visible on the surface noted at the end of cuticle reticulation (microchannels) (indicated by arrows)
Lip
The dark red thick lip was arched, with adaxial central longitudinal groove from the base to the apex (Fig. 6a). The epidermal cells on groove were leaned and arranged imbricately towards the apex (Fig. 6d). On the lobes at the lip base (Fig. 6a) occurred conical papillae with undulated longitudinal cuticular striations (Fig. 6c). The remnants of substances were visible in the groove (Fig. 6e). On the cross-section of the fleshy lip (Fig. 6b), single-layer epidermis, few layer subepidermal cells and parenchyma with several collateral vascular bundles and intracellular spaces were visible. In epidermis and subepidermis, numerous idioblasts with raphides, but no starch grains were noted (Fig. 6b). The epidermal cells from the groove and lobes on the adaxial surface were more intensively stained on proteins (Fig. 6f) in comparison with parenchyma cells and epidermis on abaxial surface. The idioblasts with raphides were present in subepidermis, and fine lipid bodies were noted in the entire lip (Fig. 6g). TEM studies revealed the presence of exudates in the longitudinal groove (Fig. 7a–d). The exudates were observed on the whole cell surface (Fig. 7a–c), sometimes on the surface above the junction of cell walls between two adjoining epidermal cells (Fig. 7d). In the cytoplasm, vesicles building into plasmalemma, abundant rough endoplasmic reticulum (rER), free ribosomes, lipid bodies close to numerous mitochondria, fully developed dictyosomes, sometimes myelin-like figures and multivesicular body were noted (Fig. 7a–d). The large vacuole contained tannin-like materials (Fig. 7b, d).
SEM and LM images of lip a, b adaxial (ventral, ad) surface with central longitudinal groove and papillate lobes (lo), ab abaxial surface; b cross-section of the lip base with single-layer epidermis, few layers of subepidermis and parenchyma with several collateral vascular bundles (vb) and intracellular spaces, note the absence of starch grains (PAS); c magnification of a, conical papillae with undulated longitudinal cuticular striations present on the lobes (SEM); d magnification of a, cells leaned and arranged imbricately towards the apex (SEM); e the remnants of substances visible in the lip groove (SEM); f papillae on lobes intensively stained on proteins (ABB); g idioblasts with raphides (r) present in subepidermis and fine lipid bodies noted in the entire lip (SBB)
TEM images of lip groove a–d exudates (black arrows) present on the epidermis, sometimes on the surface above the junction of cell walls (cw) between two adjoining epidermal cells visible on d. In the cytoplasm present vesicles (ve) building into plasmalemma (pl), lipid bodies (l) close to mitochondria (m), fully developed dictyosomes (d), free ribosomes (ri), myelin-like figures (white arrows on c, d) and multivesicular body (mvb on c); LM and TEM images of stelidia (st) on gynostemium e gynostemium (g) with anther (a), stelidia (st), wings (w) and prolonged column foot (cf) and hinged lip, lip with lobes (lo) (LM); f cross-section of stelidium with cells with strongly undulated cuticle (ABB); g vacuole (va) and parietal layer of cytoplasm with rough endoplasmic reticulum (rER), mitochondria (m), plastid (p). Thick cell wall (cw) covered by cuticle consisted of slightly reticulate cuticle layer (cl) and cuticle proper (cp) with no signs of secretory activity on the surface (TEM); h magnification of cell wall (cw), cuticle layer (cl) and cuticle proper (cp). No signs of secretory activity on the surface (TEM)
Gynostemium
Gynostemium is erect, apically incurved with wings, long, narrow stelidia and prolonged column foot (Fig. 7e). The stelidia were built up of the cells with strongly undulated cuticle (Fig. 7f–h), but no exudates (proteins, polysaccharides, lipids) were noticed on their surface. TEM observations indicated thick cell wall with cuticle consisted of slightly reticulate cuticle layer, but with no signs of secretory activity on the surface of cuticle proper (Fig. 7h). The parietal layer of cytoplasm contained mitochondria, rough endoplasmic reticulum (rER) and plastids (Fig. 7g).
Discussion
The flowers of Bulbophyllum wendlandianum fulfil features that characterize fly-pollinated sapromyophilous flowers, such as floral colours, the presence of motile appendages and see-saw lip. The appendages of the dorsal sepal and petals function as osmophores. Synthesis of fragrance components could occur in plastids in plastoglobuli. The intraplastidal membranes, plastid envelope and ER are in close vicinity, so the compounds could be transported via ER to plasmalemma or independently as lipophilic or osmiophilic droplets in cytoplasm (Pridgeon and Stern 1985; Stern et al. 1987; Pais and Figueiredo 1994; Stpiczyńska 1997; Kowalkowska et al. 2012). There were no pores or cracks observed on the epidermal epithelial cells of osmophore surface, similarly to other orchid osmophores (Pridgeon and Stern 1985; Stern et al. 1987). How could secretion be exuded through the uninterrupted cuticle layer and cuticle proper covering the outer tangential walls? In our view, the exudation is transported inside vesicles via granulocrine secretion and, when the vesicles are building into plasmalemma, they gather between the plasmalemma and cell wall. Presence of the irregular plasmalemma, with vesicles building into it, connected with granulocrine secretion was previously described in fragrant Gymnadenia and Anacamptis) (Stpiczyńska 2001; Kowalkowska et al. 2012). The cuticle reticulation functions as microchannels. It seems to be that the transport of substances through cuticle proper outside is possible because in some places at the end of microchannels the exudates were visible. The presence of cuticle with microchannels involved in transfer of fragrance compounds is described in Passiflora suberosa L. (García et al. 2007), Orbea variegata L. (Płachno et al. 2010) and Anacamptis pyramidalis f. fumeauxiana (Kowalkowska et al. 2012). The cuticle proper seems to be completely permeable. It stretches and forms swellings on the entire cell surface, sometimes at the points between adjoining epidermal cells (as it is in Maxillaria coccinea, Stpiczyńska et al. 2004). The frequently appeared cuticle swellings on the appendages are similar to those previously described on the surface of a nectary in M. coccinea (Stpiczyńska et al. 2004). These ones are up to 2 µm high, whereas in M. coccinea up to 7 µm high. They are also morphologically similar to the papillae on radii of Passiflora suberosa (García et al. 2007), but ultrastructurally they are different. In P. suberosa, papillae are formed from cell wall, covered by thin layer of cuticle. In B. wendlandianum, the swellings are formed from cuticle. On the surface of the appendages, a few amount of lipids was present, with meagre accumulation, which also confirms the osmophore function. Fragrances may be mixtures of many constituents and usually are produced and released periodically (Vogel 1990) without accumulation on the surface. Meagre amount of accumulated secretory remains were detectable in Stanhopea (Stern et al. 1987; Vogel 1990) and Anacamptis (Kowalkowska et al. 2012). Lipids, which are appeared to be the equivalents of fragrance production, were observed in other orchids (Swanson et al. 1980; Pridgeon and Stern 1983; Curry et al. 1988). Such fragrance volatilization by cuticular diffusion was previously described in other orchids (Vogel 1990; Stern et al. 1987; Curry et al. 1988; Stpiczyńska 1993, 2001), while in species of Restrepia and Restrepiella (Pridgeon and Stern 1983) fragrance is emitted through cuticular pores or in Acianthera via stomata (Melo et al. 2010). Nevertheless, for, i.e. terpenoids—highly lipophilic compounds, the volatilization by cuticle diffusion is preferred than through stomata (Riederer 2006). The common feature of osmophore tissue is the accumulation of starch grains in amyloplasts during pre-secretory stage and its hydrolization at the anthesis stage (Stern et al. 1987; Curry et al. 1991; Melo et al. 2010; Pansarin et al. 2009; Antoń et al. 2012). The starch is exploited as a source of energy in fragrance production (Vogel 1990). Nevertheless, in some fragrant orchids as in Cypripedium (Swanson et al. 1980), Gymnadenia conopsea (Stpiczyńska 2001), Cyclopogon elatus (Wiemer et al. 2009) and Anacamptis pyramidalis f. fumeauxiana (Kowalkowska et al. 2012), the plastids are starchless, as in the appendages of Bulbophyllum wendlandianum. The starchless plastids in Cypripedium were produced and emitted lipid soluble odours (Swanson et al. 1980). The plastoglobuli joined with intraplastidal membranes noted in plastids were typical for osmophore cells observed in Citrus deliciosa (Bosabalidis and Tsekos 1982), Pinus (Fahn 1988), Platanthera bifolia (Stpiczyńska 1997), Gymnadenia conopsea (Stpiczyńska 2001). The lack of starch in appendages of Bulbophyllum wendlandianum could also be caused by their hydrolization during anthesis, as our research concerned only flowers in anthesis, not the pre-secretory stage. However, it is less possible, as in this stage starch grains were detected in the cells of apices of lateral sepals. On the other hand, some plastids are elongated, which could be caused by depletion of starch grains and development of plastoglobuli (Sawidis 1998). The large number of idioblasts with raphides of calcium oxalate was observed in tepals. Idioblasts with raphides have been noted in tepals of other orchid species (Stpiczyńska et al. 2004, 2005a; Kowalkowska and Margońska 2009), often accompanied by the secretory cells (nectaries, resin glands, elaiophores) (Stpiczyńska et al. 2007, 2011; Davies and Stpiczyńska 2012). Paiva and Machado (2008) claimed that the presence of idioblasts might be related to the exclusion of additional calcium from the cytosol.
Nectaries in Orchidaceae can be located in long spurs produced from the base of lip or from the joined sepals (mentum), as tubes embedded in the ovary or on the side-lobes of the lip along the central groove of the lip (van der Pijl and Dodson 1969). In Bulbophyllum, nectar is usually exposed superficially in lip grooves (van der Pijl and Dodson 1969; Vogel 1990; Borba and Semir 1998; Kowalkowska 2009). In Bulbophyllum wendlandianum, the nectary comprises a secretory epidermis and few subepidermal layers and is located in central groove on the adaxial surface. The dense cytoplasm contains abundant mitochondria, numerous ER, frequent fully developed dictyosomes, free ribosomes, lipid bodies, multivesicular body and myelin-like figures. In large vacuole, tannin-like materials were detected. The profusion of mitochondria in nectariferous or osmophoric tissues has been reported previously (Pridgeon and Stern 1983; Stpiczyńska et al. 2005b) and is connected with high metabolic cell activity. The profuse ER and fully developed dictyosomes are involved in nectar secretion (Figueiredo and Pais 1992; Stpiczyńska et al. 2005a). In orchid nectaries cells, osmiophilic substances (presumably lipid bodies) are frequently noted (Stpiczyńska 1997; Stpiczyńska et al. 2004; Pais and Figueiredo 1994). The lipid bodies occurred in epidermal cells of all lip surface in B. rothschildianum (Teixeira et al. 2004). In our results, the lipid bodies were placed close to mitochondria, which can be connected with secretory process. The lack of starch could be caused by its hydrolisation or, the same as in nectaries of other orchids, starch do not accumulate during any secretory stage (Stpiczyńska et al. 2004). The vesicles building into plasmalemma were noted frequently, which could indicate transport of volatile and/or nectar components (Fahn 1988, also described above). The large amount of exudates was noted directly on the cell wall, sometimes also at the points between adjoining cells, but generally exudation was visible on the whole surface of cell wall. It seems that the secretion is transported in granulocrine secretion, the same as on the appendages. Bulbophyllum wendlandianum in its general floral appearance resembles the B. ornatissimum and B. rothschildianum in colour, motile lips with central groove and appendages. The same as in B. rothschildianum, lip consists of papillate lobes, but not prolonged to unicellular trichomes (Teixeira et al. 2004). In B. ornatissimum, Vogel (1990) described two heterogenous fragrance centres: the tail osmophores with cod-liver oil or fish odour and the trimethylamine odour released from lip surface. The ABB stain of lip proved that the adaxial epidermis contains more proteins than parenchyma cells. Sapromyophilous flowers generally emanate odours similar to proteinaceous compound in decomposition, mostly amines, ammonia and indoles (Proctor et al. 1996). In our opinion, the superficially gathered secretion is nectar. Nectar composition could vary widely, depending on the insects attracted to flowers. Ingredients dissolved in nectar function variously: rewarding pollinators with water, carbohydrates, amino acids, ions and low molecular weight proteins, also containing fragrant compounds to entice consumers (Raguso 2004) and enzymes and antioxidants sustaining homoeostasis of nectar composition (Carter and Thornburg 2004). Moreover, it may also comprise toxic materials to discourage unwanted consumers—non-visitors (Adler 2001). The papillate lobes on lip could function as osmophores as in some Neotropical Bulbophyllum species (Teixeira et al. 2004), but in B. wendlandianum we did not notice histological or ultrastructural differences between papillae and labellar groove. The whole adaxial lip epidermis revealed the secretory features.
The tannin-like material found in cells of studied tepals offers protection against pathogens, herbivores and UV radiation. Tannins are described in cell suspension cultures and calluses from various gymnosperms (Constabel 1969; Chafe and Durzan 1973; Parham and Kaustinen 1977) or in petals (Ochir et al. 2010), forming in tannosomes (Brillouet et al. 2013). The stelidia, with no secretion on the very undulated cuticle, do not emit fragrance.
In conclusion, in Bulbophyllum wendlandianum the nectary occur in central longitudinal groove on the adaxial lip surface comprised of a secretory epidermis and few subepidermal layers. The osmophore activity is in highest probability present on appendages of dorsal sepal and petals, but chemical analysis may confirm the presence of different types of exudates produced on appendages and on lip groove. The swellings of cuticle on the appendages of dorsal sepal and petals were not previously described in Bulbophyllum species.
References
Adler LS (2001) The ecological significance of toxic nectar. Oikos 91:409–420
Antoń S, Kamińska M, Stpiczyńska M (2012) Comparative structure of the osmophores in the flower of Stanhopea graveolens L. and Cycnoches chlorochilon Klotzsch (Orchidaceae). Acta Agrobot 65:11–22
Augustine J, Kumar Y (2001) Orchids of India II: biodiversity and status of Bulbophyllum Thou. Daya Publishing House, Delhi
Azevedo MTA, Borba EL, Semir J, Solferini VN (2007) High genetic variability in Neotropical myophilous orchids. Bot J Linn Soc 153:33–40
Borba EL, Semir J (1998) Wind-assisted fly pollination in three Bulbophyllum (Orchidaceae) species occurring in the Brazilian campos rupestres. Lindleyana 13:203–218
Bosabalidis A, Tsekos I (1982) Ultrastructural studies on the secretory cavities of Citrus deliciosa Ten. I. early stages of the gland cell differentiation. Protoplasma 112:55–62
Brillouet J-M, Romieu C, Schoefs B, Solymosi K, Cheynier V, Fulcrand H, Verdeil J-L, Conéjéro G (2013) The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann Bot. doi:10.1093/aob/mct168
Bronner R (1975) Simultaneous demonstration of lipid and starch in plant tissues. Stain Tech 50(1):1–4
Carter C, Thornburg RW (2004) Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci 9:320–324
Chafe SC, Durzan DJ (1973) Tannin inclusions in cell suspension cultures of white spruce. Planta 113:251–262
Chowlu K, Rao AN, Angela N, Vij SP (2013) A brief account of the genus Bulbophyllum (Orchidaceae) in Manipur, India. Rheedea 23:86–97
Christensen DE (1994) Fly pollination in the Orchidaceae. In: Arditti J (ed) Orchid biology: reviews and perspectives, vol VI. Wiley, New York, pp 415–454
Comber JB (1990) Orchids of Java. Bentham-Moxon Trust, Kew
Comber JB (2001) Orchids of Sumatra. Natural History Publications, Kota Kinabalu
Constabel F (1969) Über die Entwicklung von Gerbstoffzellen in Calluskulturen von Juniperus communis L. Planta Med 17:101–115
Curry KJ, Stern WL, McDowell LM (1988) Osmophore development in Stanhopea anfracta and S. pulla (Orchidaceae). Lindleyana 3:212
Curry KJ, McDowell LM, Judd WS, Stern WL (1991) Osmophores, floral features, and systematics of Stanhopea (Orchidaceae). Am J Bot 78:610–623
Davies KL, Stpiczyńska M (2012) Comparative labellar anatomy of resin-secreting and putative resin-mimic species of Maxillaria s.l. (Orchidaceae: Maxillariinae). Bot J Linn Soc 170:405–435
Fahn A (1988) Secretory tissues in vascular plants. New Phytol 108:229–257
Feder N, O’Brien TP (1968) Plant microtechnique; some principles and new methods. Am J Bot 55:123–142
Figueiredo ACS, Pais MS (1992) Ultrastructural aspects of the nectary spur of Limodorum abortivum (L.) Sw Orchidaceae. Ann Bot 70:325–331
García MTA, Galati BG, Hoc PS (2007) Ultrastructure of the corona of scented and scentless flowers of Passiflora spp. (Passifloraceae). Flora 202:302–315
Hosseini S (2011) Morphological and molecular systematics of Bulbophyllum Thou, in Peninsular Malaysia. PhD thesis, Universiti Putra Malaysia
Hosseini S, Go R, Dadkhah K, Ainuddin Nuruddin A (2012) Studies on maturase K sequences and systematic classification of Bulbophyllum in Peninsular Malaysia. Pak J Bot 44:2047–2054
Jensen WA (1962) Botanical Histochemistry. Freeman, San Francisco
Jones DL, Gray B (1976) The pollination of Bulbophyllum longiflorum Thouars. Amer Orchid Soc Bull 45:15–17
Jongejan P (1994) Specializations in ways of attracting insects for pollination in the genus Bulbophyllum. In: Proceedings of 14th WOC, HMSO, Glasgow, pp 383–388
Knerr JN (1981) The genus Bulbophyllum: a living phantasy. Amer Orchid Soc Bull 50:1051–1056
Kowalkowska AK (2009) Analiza porównawcza struktur kwiatowych wabiących owady u wybranych gatunków Bulbophyllinae Schltr. i Pleurothallidinae Lindl. (Orchidaceae)/Comparative analysis of floral structures attracting insects in selected species of Bulbophyllinae Schltr. and Pleurothallidinae Lindl. (Orchidaceae). Dissertation, University of Gdańsk
Kowalkowska AK, Margońska HB (2009) Diversity of labellar micromorphological structures in selected species of Malaxidinae (Orchidales). Acta Soc Bot Pol 78:141–150
Kowalkowska AK, Margońska HB, Kozieradzka-Kiszkurno M (2010) Comparative anatomy of the lip spur and additional lateral sepal spurr in a three-spurred form (f. fumeauxiana) of Anacamptis pyramidalis. Acta Biol Cracov Ser Bot 52:13–18
Kowalkowska AK, Margońska HB, Kozieradzka-Kiszkurno M, Bohdanowicz J (2012) Studies on the ultrastructure of a three-spurred fumeauxiana form of Anacamptis pyramidalis. Plant Syst Evol 298:1025–1035
Kozieradzka-Kiszkurno M, Płachno BJ (2013) Diversity of plastid morphology and structure along the micropyle-chalaza axis of different Crassulaceae. Flora 208:128–137
Luer CA (1982) Condylago, un nuevo genero en las Pleurothallidinae. Condylago, a new genus in the Pleurothallidinae. Orquideología 15:117–122
Luer CA (1987) Icones Pleurothallidinarum IV: systematics of Acostaea, Condylago, and Porroglossum (Orchidaceae). Monogr Syst Bot Mo Bot Gard 24:1–91
Melo MC, Borba EL, Paiva EAS (2010) Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae). Plant Syst Evol 286:141–151
Merino G, Doucette A, Pupulin F (2010) New species of Porroglossum (Orchidaceae: Pleurothallidinae) from Ecuador. Lankesteriana 9:459–466
Ochir S, Park B, Nishizawa M, Kanazawa T, Funaki M, Yamagishi TJ (2010) Simultaneous determination of hydrolysable tannins in the petals of Rosa rugosa and allied plants. Nat Med 64:383–387
Ong PT (2011a) The pollination of Bulbophyllum patens. Orchid Rev 119:146–149
Ong PT (2011b) The importance of Bactrocera fruit flies as pollinators of Bulbophyllum orchids. Conserv Malaysia 14:4–5
Ong PT, Tan KH (2012) Three species of Bulbophyllum section Racemosae pollinated by Drosophila flies. Malesian Orchid J 9:45–50
Ong PT, O’Byrne P, Yong WSY, Saw LG (2011a) Wild Orchids of Peninsular Malaysia. Forest Research Institute, Malaysia
Ong PT, Hee AKW, Wee SL, Tan KH (2011b) The attraction of flowers of Bulbophyllum (Section Sestochilus) to Bactrocera Fruit Flies (Diptera: Tephritidae). Malesian Orchid J 8:93–102
Ossian CR (1983) Noteworthy bulbophyllums and cirrhopetalums. I. Large-flowered, umbellate forms. Amer Orchid Soc Bull 52:108–117
Pais MS, Figueiredo ACS (1994) Floral nectaries from Limodorum abortivum (L.) Sw. and Epipactis atropurpurea Rafin (Orchidaceae): ultrastructural changes in plastids during the secretory process. Apidologie 25:615–626
Paiva EAS, Machado SR (2008) The floral nectary of Hymenaea stigonocarpa (Fabaceae, Caesalpinioideae): structural aspects during floral development. Ann Bot 101:125–133
Pansarin LM, Castro M, Sazima M (2009) Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Bot J Linn Soc 159:408–415
Parham RA, Kaustinen HM (1977) On the site of tannin synthesis in plant cells. Bot Gaz 138:465–467
Pearce NR, Cribb PJ (2002) Orchids of Bhutan. Royal Botanic Gardens Edinburgh
Płachno BJ, Świątek P, Szymczak G (2010) Can a stench be beautiful? Osmophores in stem-succulent stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae-Stapeliinae). Flora 205:101–105
Pridgeon AM, Stern WL (1983) Ultrastructure of osmophores in Restrepia (Orchidaceae). Am J Bot 70:1233–1243
Pridgeon AM, Stern WL (1985) Osmophores of Scaphosepalum (Orchidaceae). Bot Gaz 146:115–123
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins Publishers, London
Raguso RA (2004) Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Curr Opin Plant Biol 7:434–440
Rao AN (2010) Orchid flora of Arunachal Pradesh—an update. Bull Arunachal For Res 26:82–110
Ridley HN (1885) The orchids of Madagascar. Bot J Linn Soc 21:456–522. doi:10.1111/j.1095-8339.1885.tb00573.x
Ridley HN (1890) On the methods of fertilization in Bulbophyllum macranthum, and allied orchids. Ann Bot 4:327–336
Riederer M (2006) Biology of the plant cuticle. In: Riederer M, Müller C (eds) Biology of the plant cuticle. Blackwell, Oxford
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Sawidis T (1998) The subglandular tissue of Hibiscus rosa-sinensis nectaries. Flora 193:327–335
Seidenfaden G (1979) Orchid genera in Thailand VIII. Dansk Bot Arkiv 33:1–228
Seidenfaden G, Wood JJ (1992) Orchids of Peninsular Malaysia and Singapore. Olsen & Olsen, Fredensborg
Sieder A, Rainer H, Kiehn M (2007) CITES orchid checklist volume 5: Bulbophyllum. http://www.cites.org/common/com/nc/tax_ref/Bulbophyllum.pdf
Sieder A, Rainer H, Kiehn M (2010) CITES orchid checklist volume 5: Bulbophyllum. Royal Botanic Gardens, Kew
Silva UF, Borba EL, Semir J (1999) A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry 50:31–34
Singer RB, Cocucci AA (1999) Pollination mechanism in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14:47–56
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastructure Res 26:31–43
Stern WL, Curry KJ, Pridgeon AM (1987) Osmophores of Stanhopea (Orchidaceae). Am J Bot 74:1323–1331
Stpiczyńska M (1993) Anatomy and ultrastructure of osmophores of Cymbidium tracyanum Rolfe (Orchidaceae). Acta Soc Bot Pol 62:5–9
Stpiczyńska M (1997) The structure of nectary of Platanthera bifolia L. (Orchidaceae). Acta Soc Bot Pol 62:5–9
Stpiczyńska M (2001) Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Soc Bot Pol 70:91–96
Stpiczyńska M, Davies KL, Gregg A (2004) Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Ann Bot 93:87–95
Stpiczyńska M, Davies KL, Gregg A (2005a) Comparative account of nectary structure in Hexisea imbricata (Lindl.) Rchb.f. (Orchidaceae). Ann Bot 95:749–756
Stpiczyńska M, Milanesi C, Faleri C, Cresti M (2005b) Ultrastructure of the nectary spur of Platanthera chlorantha (Custer) Rchb. (Orchidaceae) during successive stages of nectar secretion. Acta Biol Crac 47:111–119
Stpiczyńska M, Davies KL, Gregg A (2007) Elaiophore diversity in three contrasting members of Oncidiinae (Orchidaceae). Bot J Linn Soc 155:135–148
Stpiczyńska M, Davies KL, Kamińska M (2011) Comparative structure of the nectary spur in selected species of Aeridinae (Orchidaceae). Ann Bot 107:327–345
Swanson ES, Cunningham WP, Holman RT (1980) Ultrastructure of glandular ovarian trichomes of Cypripedium calceolus and C. reginae (Orchidaceae). Amer J Bot 67:784–789
Tan KH (2008) Fruit fly pests as pollinators of wild orchids. In: Fruit flies of economic importance: from basic to applied knowledge—Proceedings of the 7th International symposium on fruit flies of economic importance, 10–15 September 2006, Salvador, Brazil, pp 195–206
Tan K, Nishida R (2000) Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J Chem Ecol 26:533–546
Tan K, Nishida R (2007) Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem Syst Ecol 35:334–341
Teixeira S, Borba EL, Semir J (2004) Lip anatomy and its implications for the pollination mechanisms of Bulbophyllum species (Orchidaceae). Ann Bot 93:499–505
van der Cingel NA (ed) (1995) An atlas of Orchid Pollination. Balkema, Rotterdam
van der Pijl L, Dodson CH (1969) Orchid flowers: their pollination and evolution. University of Miami Press, Coral Gables
Vermeulen JJ (1991) Orchids of Borneo: Bulbophyllum II. Bentham-Moxon Trust, Royal Botanic Gardens, Kew
Vermeulen JJ (2008) New species of Bulbophyllum from eastern Malesia (Orchidaceae). Nord J Bot 26:129–195
Vogel S (1990) The role of scent glands in pollination: on the structure and function of osmophores. Amerind, New Delhi
Vogel S (2001) Flickering bodies: floral attraction by movement. Beitr Biol Pflanzen 72:89–154
Wiemer AP, More M, Benitez-Vieyra S, Cocucci AA, Raguso RA, Sersic AN (2009) A simple floral fragrance and unusual osmophore structure in Cyclopogon elatus (Orchidaceae). Plant Biol 11:506–514
Acknowledgments
This work was supported by the National Science Centre in Poland (5804/B/PO1/2010/39). The first author is very grateful to Univ.-Prof. Dr. Michael Kiehn and Anton Sieder from Botanischer Garten der Universität Wien for good cooperation with Bulbophyllinae studies. We thank Rudolf Hromniak for sharing the photo for this article. We also thank the anonymous reviewers for helpful commentary on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kowalkowska, A.K., Kozieradzka-Kiszkurno, M. & Turzyński, S. Morphological, histological and ultrastructural features of osmophores and nectary of Bulbophyllum wendlandianum (Kraenzl.) Dammer (B. section Cirrhopetalum Lindl., Bulbophyllinae Schltr., Orchidaceae). Plant Syst Evol 301, 609–622 (2015). https://doi.org/10.1007/s00606-014-1100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1100-2