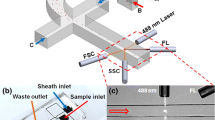
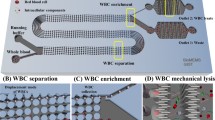
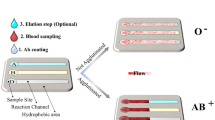
Abstract
White blood cells (WBCs) are robust defenders during antigenic challenges and prime immune cell functioning indicators. High-purity WBC separation is vital for various clinical assays and disease diagnosis. Red blood cells (RBCs) are a major hindrance in WBC separation, constituting 1000 times the WBC population. The study showcases a low-cost micropump integrated microfluidic platform to provide highly purified WBCs for point-of-care testing. An integrated user-friendly microfluidic platform was designed to separate WBCs from finger-prick blood (⁓5 μL), employing an inertial focusing technique. We achieved an efficient WBC separation with 86% WBC purity and 99.99% RBC removal rate in less than 1 min. In addition, the microdevice allows lab-on-chip colorimetric evaluation of chronic granulomatous disease (CGD), a rare genetic disorder affecting globally. The assay duration, straight from separation to disease detection, requires only 20 min. Hence, the proposed microfluidic platform can further be implemented to streamline various clinical procedures involving WBCs in healthcare industries.
Graphical abstract







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data supporting the findings of this study are available from the corresponding author on request.
References
Manouk AAV (2019) Dynamics of blood cell suspensions in microflows. CRC Press, Boca Raton
Prinyakupt J, Pluempitiwiriyawej C (2015) Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed Eng Online 14:63. https://doi.org/10.1186/s12938-015-0037-1
Langer S, Radhakrishnan N, Pradhan S et al (2021) Clinical and laboratory profiles of 17 cases of chronic granulomatous disease in North India. Indian J Hematol Blood Transfus 37:45–51. https://doi.org/10.1007/s12288-020-01316-6
George A, Pushkaran S, Konstantinidis DG et al (2013) Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 121:2099–2107. https://doi.org/10.1182/blood-2012-07-441188
Buvelot H, Posfay-Barbe KM, Linder P et al (2017) Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev 41:139–157. https://doi.org/10.1093/femsre/fuw042
Civelekoglu O, Ozkaya-Ahmadov T, Arifuzzman AKM et al (2022) Immunomagnetic leukocyte differential in whole blood on an electronic microdevice. Lab Chip 22:2331–2342. https://doi.org/10.1039/D2LC00137C
Rider NL, Jameson MB, Creech CB (2018) Chronic granulomatous disease: epidemiology, pathophysiology, and genetic basis of disease. J Pediatr Infect Dis Soc 7:S2–S5. https://doi.org/10.1093/jpids/piy008
Rook GAW, Steele J, Umar S, Dockrell HM (1985) A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J Immunol Methods 82:161–167. https://doi.org/10.1016/0022-1759(85)90235-2
Sim Choi H, Woo Kim J, Cha Y, Kim C (2006) A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem 27:31–44. https://doi.org/10.1080/15321810500403722
Ossowski P, Raiter-Smiljanic A, Szkulmowska A et al (2015) Differentiation of morphotic elements in human blood using optical coherence tomography and a microfluidic setup. Opt Express, OE 23:27724–27738. https://doi.org/10.1364/OE.23.027724
Nam J, Yoon J, Kim J et al (2019) Continuous erythrocyte removal and leukocyte separation from whole blood based on viscoelastic cell focusing and the margination phenomenon. J Chromatogr A 1595:230–239. https://doi.org/10.1016/j.chroma.2019.02.019
Chiu P-L, Chang C-H, Lin Y-L et al (2019) Rapid and safe isolation of human peripheral blood B and T lymphocytes through spiral microfluidic channels. Sci Rep 9:8145. https://doi.org/10.1038/s41598-019-44677-3
Shin HS, Park J, Lee SY et al (2023) Integrative magneto-microfluidic separation of immune cells facilitates clinical functional assays. Small 19:2302809. https://doi.org/10.1002/smll.202302809
Xu X, Huang X, Sun J et al (2021) Recent progress of inertial microfluidic-based cell separation. Analyst 146:7070–7086. https://doi.org/10.1039/D1AN01160J
Hassan U, Ghonge T, Reddy B Jr et al (2017) A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat Commun 8:15949. https://doi.org/10.1038/ncomms15949
Lee S, Kim H, Yang S (2023) Microfluidic label-free hydrodynamic separation of blood cells: recent developments and future perspectives. Adv Mater Technol 8:2201425. https://doi.org/10.1002/admt.202201425
Matsuura K, Takata K (2023) Blood cell separation using polypropylene-based microfluidic devices based on deterministic lateral displacement. Micromachines (Basel) 14:238. https://doi.org/10.3390/mi14020238
Kim B, Kim KH, Chang Y et al (2019) One-step microfluidic purification of white blood cells from whole blood for immunophenotyping. Anal Chem 91:13230–13236. https://doi.org/10.1021/acs.analchem.9b03673
Pamme N (2007) Continuous flow separations in microfluidic devices. Lab Chip 7:1644–1659. https://doi.org/10.1039/B712784G
Bakhshi MS, Rizwan M, Khan GJ et al (2022) Design of a novel integrated microfluidic chip for continuous separation of circulating tumor cells from peripheral blood cells. Sci Rep 12:17016. https://doi.org/10.1038/s41598-022-20886-1
Zhu Z, Wu D, Li S et al (2021) A polymer-film inertial microfluidic sorter fabricated by jigsaw puzzle method for precise size-based cell separation. Anal Chim Acta 1143:306–314. https://doi.org/10.1016/j.aca.2020.11.001
Kwon T, Prentice H, Oliveira JD et al (2017) Microfluidic cell retention device for perfusion of mammalian suspension culture. Sci Rep 7:6703. https://doi.org/10.1038/s41598-017-06949-8
Zhang J, Yuan D, Sluyter R et al (2017) High-throughput separation of white blood cells from whole blood using inertial microfluidics. IEEE Trans Biomed Circuits Syst 11:1422–1430. https://doi.org/10.1109/TBCAS.2017.2735440
Wu L, Guan G, Hou HW et al (2012) Separation of leukocytes from blood using spiral channel with trapezoid cross-section. Anal Chem 84:9324–9331. https://doi.org/10.1021/ac302085y
Mehran A, Rostami P, Saidi MS et al (2021) High-throughput, label-free isolation of white blood cells from whole blood using parallel spiral microchannels with U-shaped cross-section. Biosensors 11:406. https://doi.org/10.3390/bios11110406
Jeon H, Jundi B, Choi K et al (2020) Fully-automated and field-deployable blood leukocyte separation platform using multi-dimensional double spiral (MDDS) inertial microfluidics. Lab Chip 20:3612–3624. https://doi.org/10.1039/D0LC00675K
Lombodorj B, Tseng HC, Chang H-Y et al (2020) High-throughput white blood cell (leukocyte) enrichment from whole blood using hydrodynamic and inertial forces. Micromachines (Basel) 11:275. https://doi.org/10.3390/mi11030275
Mane S, Hemadri V, Tripathi S (2023) Investigating WBC margination in different microfluidic geometries: influence of RBC shape and size. J Micromech Microeng 33:065002. https://doi.org/10.1088/1361-6439/acca29
Wu Z, Chen Y, Wang M, Chung AJ (2016) Continuous inertial microparticle and blood cell separation in straight channels with local microstructures. Lab Chip 16:532–542. https://doi.org/10.1039/C5LC01435B
Bhattad S (2022) Primary Immune Deficiencies Made Simple: Handbook for Practicing Pediatricians. CBS Publishers & Distributors, Physicians and Medical Students
Zhao Q, Yuan D, Zhang J, Li W (2020) A review of secondary flow in inertial microfluidics. Micromachines 11:461. https://doi.org/10.3390/mi11050461
Mane S, Hemadri V, Tripathi S (2022) Separation of white blood cells in a wavy type microfluidic device using blood diluted in a hypertonic saline solution. BioChip J 16:291–304. https://doi.org/10.1007/s13206-022-00074-z
Nivedita N, Ligrani P, Papautsky I (2017) Dean Flow Dynamics in low-aspect ratio spiral microchannels. Sci Rep 7:44072. https://doi.org/10.1038/srep44072
Martel JM, Toner M (2014) Inertial focusing in microfluidics. Annu Rev Biomed Eng 16:371–396. https://doi.org/10.1146/annurev-bioeng-121813-120704
Zhou J, Papautsky I (2013) Fundamentals of inertial focusing in microchannels. Lab Chip 13:1121–1132. https://doi.org/10.1039/C2LC41248A
Liu N, Petchakup C, Tay HM et al (2019) Spiral inertial microfluidics for cell separation and biomedical applications. In: Tokeshi M (ed) Applications of microfluidic systems in biology and medicine. Springer, Singapore, pp 99–150. https://doi.org/10.1007/978-981-13-6229-3_5
Özbey A, Karimzadehkhouei M, Akgönül S et al (2016) Inertial focusing of microparticles in curvilinear microchannels. Sci Rep 6:38809. https://doi.org/10.1038/srep38809
Warkiani ME, Guan G, Luan KB et al (2013) Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 14:128–137. https://doi.org/10.1039/C3LC50617G
Acknowledgements
The authors acknowledge the technical support for manufacturing microchannel mold from Mr. Hemeshwar, Department of Mechanical Engineering, Bits Pilani K K Birla Goa Campus. We want to acknowledge the Department of Biological Sciences, BITS-Pilani, K. K. Birla Goa campus for BD-FACS Cell Sorter equipment sponsored by DST-FIST for sample analysis under the assistance of Mr. Tushar Honwarkar.
Funding
This work was supported by BITS BioCyTiH foundation under grant BBF/BITS (G)/FY2022-23/BCPS-115, covered by National Mission for Interdisciplinary- Cyber Physical System (NM-ICPS) of Department of Science and Technology, Government of India (D.O: DST/NM-ICPS/MGB/2018).
Author information
Authors and Affiliations
Contributions
Sanjay Mane: conceptualization, methodology, writing—original draft preparation. Abhishek Behera: investigation, data curation, visualization, writing—original draft preparation. Vadiraj Hemadri: formal analysis, investigation. Sunil Bhand: resources, funding acquisition. Siddhartha Tripathi: conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Human Ethical Committee, Bits-Pilani, K K Birla Goa Campus (EC Registration No.: EC/NEW/INST/2023/3297) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All blood samples were tested for infectious illnesses before being used.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 772 KB)
Supplementary file2 (AVI 6873 KB)
Supplementary file3 (AVI 2714 KB)
Supplementary file4 (AVI 6052 KB)
Supplementary file5 (AVI 10286 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mane, S., Behera, A., Hemadri, V. et al. Micropump integrated white blood cell separation platform for detection of chronic granulomatous disease. Microchim Acta 191, 295 (2024). https://doi.org/10.1007/s00604-024-06372-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06372-7