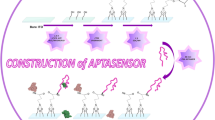
Abstract
The design of a novel electrochemical impedimetric biosensor for label-free analysis of resistin, a biomarker for obesity, is reported. For the fabrication of the immunosensor, a novel approach composed of electrochemical copolymerization of double epoxy groups-substituted thiophene (ThidEp) and 3,4-Ethylenedioxythiophene (EDOT) monomers was utilized. Anti-resistin antibodies were covalently attached to the copolymer-coated electrode. The capture of resistin antigens by anti-resistin antibodies caused significant variations in charge transfer resistance (Rct) because of the immunoreactions between these proteins. Under optimum experimental variables, the changes in impedance signals were employed for the determination of resistin antigen concentration, and the prepared immunosensor based on conjugated copolymer illustrated a wide linear range between 0.0125 and 22.5 pg/mL, a low detection limit (LOD) of 3.71 fg/mL, and a good sensitivity of 1.22 kΩ pg−1mL cm2. The excellent analytical performance of the resistin immunosensor in terms of selectivity, sensitivity, repeatability, reproducibility, storage stability, and low detection limit might be attributed to the conductive copolymer film layer generation on the disposable indium tin oxide (ITO) platform. The capability of this system for the determination of resistin in human serum and saliva samples was also tested. The immunosensor results were in accordance with the enzyme-linked immunosorbent assay (ELISA) results. The matrix effects of human serum and saliva were also investigated, and the proposed immunosensor displayed good recovery ranging from 95.91 to 106.25%. The engineered immunosensor could open new avenues for obesity monitoring.
Graphical Abstract












Similar content being viewed by others
References
Tinahones FJ, Leticia C, Maria DM et al (2012) Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Phys 12:1–8. https://doi.org/10.1186/1472-6793-12-4
Kim S, Barry MP (2006) Commentary: Understanding the epidemiology of overweight and obesity a real global public health concern Int. J. Epidemiol 35:60–67. https://doi.org/10.1093/ije/dyi255
Frank BH (2013) Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev 14:606–19. https://doi.org/10.1111/obr.12040
Matthew AS, Wieland K (2015) Childhood obesity: current and novel approaches Best Pract. Res. Clin. Endocrinol. Metab 29:327–38. https://doi.org/10.1016/j.beem.2015.04.003
Nimptsch K, Stefan K, Tobias P (2019) Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism 92:61–70. https://doi.org/10.1016/j.metabol.2018.12.006
Inci U, Mustafa KS (2022) A direct and simple immobilization route for immunosensors by CNBr activation for covalent attachment of anti-leptin: obesity diagnosis point of view. Biotech 12(1):33. https://doi.org/10.1007/s13205-021-03096-w
Tamanna I et al (2022) Detection of leptin using electrocatalyst mediated impedimetric sensing. ACS Biomater. Sci. Eng 9(5):2170–2180. https://doi.org/10.1021/acsbiomaterials.2c00642
Aleksandrova K, Dariush M, Tobias P (2018) Addressing the perfect storm: biomarkers in obesity and pathophysiology of cardiometabolic risk Clin. Chem 64:142–53. https://doi.org/10.1373/clinchem.2017.275172
Irena M et al (2012) Measurement of salivary resistin, visfatin and adiponectin levels. Peptides 33(1):120–124. https://doi.org/10.1016/j.peptides.2011.11.007
Katharina N, Tobias P, (2016) Obesity biomarkers, metabolism and risk of cancer: an epidemiological perspective Obesity and Cancer 199-217. https://doi.org/10.1007/978-3-319-42542-9_11
Hiroshi S, Sei M, Natsuko K et al (2017) Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis Arthritis Res. Ther 19:1–10. https://doi.org/10.1186/s13075-017-1472-0
Na W, Gaowa E, Yan D et al (2021) Correlation of serum resistin level and other metabolic hormones and immune function in neonatal umbilical cord blood. Medicine 100:11. https://doi.org/10.1097/MD.0000000000025195
Murat Y, Neslihan B, Hüseyin D et al (2009) Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecol. Endocrinol 25(4):246–252. https://doi.org/10.1080/09513590802653833
Israel H et al (2005) The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol 193(3):979–983. https://doi.org/10.1016/j.ajog.2005.06.041
John NF, Sean CB, Shabha HM et al (2003) Resistin release by human adipose tissue explants in primary culture. Biochem. Biophys. Res. Commun 300(3):674–678. https://doi.org/10.1016/S0006-291X(02)02864-4
Aya F, Hiroshi O, Kiyoe O et al (2004) Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clinica Chimica Acta 339(1–2):57–63. https://doi.org/10.1016/j.cccn.2003.09.009
Elif BA, Muhammet A, Mustafa KS (2023) A novel electrochemical impedance immunosensor for the quantification of CYFRA 21–1 in human serum. Microchim. Acta 190(6):235. https://doi.org/10.1007/s00604-023-05813-z
Elif B A, Muhammet A, Mustafa K S (2023) A label-free electrochemical biosensor for highly sensitive detection of GM2A based on gold nanoparticles/conducting amino-functionalized thiophene polymer layer. Sens. Actuators, B 392:134025. https://doi.org/10.1016/j.snb.2023.134025
Elif BA, Muhammet A, Mustafa KS (2021) Ultrasensitive and selective impedimetric determination of prostate specific membrane antigen based on di-succinimide functionalized polythiophene covered cost-effective indium tin oxide. Macromol. Biosci 21(10):2100173. https://doi.org/10.1002/mabi.202100173
Pan Z, Tingting S, Shengzhong R et al (2019) A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH2 nanocomposite’. Bioelectrochemistry 127:163. https://doi.org/10.1016/j.bioelechem.2019.02.003
Parastoo V, Mohammed Z, Johan B, et al (2022) A review on conjugated polymer-based electronic tongues Anal. Chim. Acta 340114. https://doi.org/10.1016/j.aca.2022.340114
Fernando RRT, Luis F (2008) Applications of polymers for biomolecule immobilization in electrochemical biosensors. Mater. Sci. Eng. C 28:1530–43. https://doi.org/10.1016/j.msec.2008.04.010
Noemi R (2009) New directions in medical biosensors employing poly (3, 4-ethylenedioxy thiophene) derivative-based electrodes. Anal. Bioanal. Chem 395:637–45. https://doi.org/10.1007/s00216-009-2981-8
Seetharamaiah N, Seetharamaiah N, Jakkid S et al (2014) Development of a simple bioelectrode for the electrochemical detection of hydrogen peroxide using Pichia pastoris catalase immobilized on gold nanoparticle nanotubes and polythiophene hybrid. Analyst 139:5800–12. https://doi.org/10.1039/C4AN01262C
Hanaa HA, Aisha AG, Mahmoud AH (2021) Polythiophene and its derivatives–based nanocomposites in electrochemical sensing: a mini review Mater. Today Commun 26
Yu L, Anthony PFT, Maojun Z, Wing CM (2018) Processable enzyme-hybrid conductive polymer composites for electrochemical biosensing. Biosens. Bioelectron 100:374–81. https://doi.org/10.1016/j.bios.2017.09.021
Ying Z (2012) Electrochemical synthesis and characterization of a novel thiazole-based copolymer and its application in biosensor. Electrochim. Acta 59:256–263. https://doi.org/10.1016/j.electacta.2011.10.061
Cheng-Yuan L, Peter F, John WB, Z, et al (2017) A urea potentiometric biosensor based on a thiophene copolymer. Biosensors 7(1):13. https://doi.org/10.3390/bios7010013
Tugce YT, Tugba S, Metin A et al (2017) Enhancing biosensor properties of conducting polymers via copolymerization: synthesis of EDOT-substituted bis (2-pyridylimino) isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron 87:81–88. https://doi.org/10.1016/j.bios.2016.08.020
Nengqin J, Qiong L, Zhiyong W et al (2009) A hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin incorporated in PEO–PPO–PEO triblock copolymer film. Sens Actuators, B 137(1):230–234. https://doi.org/10.1016/j.snb.2008.10.011
Rongli Z, Can J, Xiaoxia F et al (2017) A gold electrode modified with a nanoparticulate film composed of a conducting copolymer for ultrasensitive voltammetric sensing of hydrogen peroxide. Microchim. Acta 185:1–9. https://doi.org/10.1007/s00604-017-2564-x
Saman S, Rahul S, Amit LS et al (2005) Development of a lactate biosensor based on conducting copolymer bound lactate oxidase. Sens Actuators, B 107(2):768–772. https://doi.org/10.1016/j.snb.2004.12.016
Elif BA, Muhammet A, Mustafa KS (2021) A novel electrochemical immunosensor based on acetylene black/epoxy-substituted-polypyrrole polymer composite for the highly sensitive and selective detection of interleukin 6 Talanta, 222:121596. https://doi.org/10.1016/j.talanta.2020.121596
Pratima RS, Suman S, Nirmal P et al (2007) Application of conducting poly (aniline-co-pyrrole) film to cholesterol biosensor. J. Appl. Polym. Sci 105:3211–19. https://doi.org/10.1002/app.26198
Qiao T, Yan CY, Min ZR et al (2009) A thermally remendable epoxy resin. J Mater Chem 19:1289–96. https://doi.org/10.1039/B811938D
Elif B A, Muhammet A, Mustafa K S, (2022) Sezgintürk, Selective and ultrasensitive electrochemical immunosensing of NSE cancer biomarker in human serum using epoxy-substituted poly (pyrrole) polymer modified disposable ITO electrode. Sens. Actuators, B 306:127613. https://doi.org/10.1016/j.snb.2019.127613
Elif BA, Muhammet A, Mustafa KS (2022) Determination of calreticulin using Fe3O4@ AuNPs core-shell functionalized with PT(COOH)2 polymer modified electrode: a new platform for the impedimetric biosensing of cancer biomarkers. Sens. Actuators, B 367:132099. https://doi.org/10.1016/j.snb.2022.132099
Emma GL, Kenneth DS, Thomas CD et al (2016) Analysis of PEDOT: PSS films after sulfuric acid treatment on silicon and fused silica using FT-IR and UV-VIS MRS. Advances 1:465. https://doi.org/10.1557/adv.2016.177
Pojjawan C, Anuvat S (2013) Effect of transition metal ion-exchanged into the zeolite Y on electrical conductivity and response of PEDOT-PSS/MY composites toward SO2. Adv. Polym. Technol 32:21367. https://doi.org/10.1002/adv.21367
Chakrit S, Chanpen K, Anurat W et al (2012) Inkjet-printed graphene-PEDOT: PSS modified screen printed carbon electrode for biochemical sensing. J. Mater. Chem 22:5478–85. https://doi.org/10.1039/C2JM14005E
Tze SP, Patthara K, Sunil KA et al (2013) Detection of tumor necrosis factor (TNF-α) in cell culture medium with label free electrochemical impedance spectroscopy Sens. Actuators, B 181:494–500. https://doi.org/10.1016/j.snb.2013.02.019
Hend SM, Rabeay YH, Ashok M (2021) Electrochemical impedance spectroscopy (EIS): principles, construction, and biosensing applications Sensors 21:6578. https://doi.org/10.3390/s21196578
Anqing A, Yaping D, Li L, et al (2019) A novel electrochemical enzyme biosensor for detection of 17β-estradiol by mediated electron-transfer system Talanta 192:478-85. https://doi.org/10.1016/j.talanta.2018.09.018
Audrey S, Loïc JB, Béatrice DL (2012) Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv 30:489–511. https://doi.org/10.1016/j.biotechadv.2011.09.003
Zeynep A, Wellington MF, Ibtisam ET (2014) Cardiovascular disease detection using bio-sensing techniques. Talanta 128:177–86. https://doi.org/10.1016/j.talanta.2014.04.060
Jack AG, Jo VR, Pa M (2015) Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31(23):6267–6276. https://doi.org/10.1021/la503533g
Saumya j, Vijay D P, Andreas M, et al (2018) Regenerative, highly-sensitive, non-enzymatic dopamine sensor and impact of different buffer systems in dopamine sensing. Biosensors 8(1):9. https://doi.org/10.3390/bios8010009
Carlo DSR, Hideko Y, Mira J et al (2006) Label-free DNA detection based on modified conducting polypyrrole films at microelectrodes. Anal Chem 78(4):139–1145. https://doi.org/10.1021/ac051478u
Elba MG, Ana C, Juan JM et al (2007) Optical immunosensor for fast and sensitive detection of DDT and related compounds in river water samples. Biosens Bioelectron 22(7):1410–1418. https://doi.org/10.1016/j.bios.2006.06.016
Funding
This work was financially supported by TUBİTAK (The Scientific and Technological Research Council of Turkey), Project number: 121 Z 833.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydın, E.B., Aydın, M. & Sezgintürk, M.K. A new immunosensing platform based on conjugated Poly(ThidEp-co-EDOT) copolymer for resistin detection, a new obesity biomarker. Microchim Acta 191, 69 (2024). https://doi.org/10.1007/s00604-023-06145-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06145-8