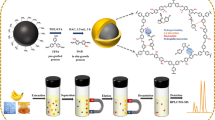
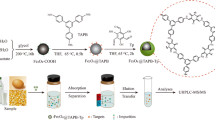
Abstract
A porous magnetic covalent organic framework, Fe3O4@TPBD-TPA (terephthalaldehyde (TPA) , N, N, N′, N′-tetrakis(p-aminophenyl)-p-phenylenediamine (TPBD)), was synthesized using the Schiff base reaction under mild reaction conditions. This adsorbent exhibited excellent adsorption performance for aflatoxins. The adsorption capacity of Fe3O4@TPBD-TPA for aflatoxins ranged from 64.4 to 84.4 mg/g. A magnetic solid-phase extraction combined with high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method based on Fe3O4@TPBD-TPA was developed for the efficient determination of four types of aflatoxins in food samples (maize, maize oil, peanut, and peanut oil). The determination coefficients (R2) were ≥0.9972. The method exhibited detection limits ranging from 0.01 to 0.06 μg/kg and spiked recoveries of 80.0 to 113.1%. The intra-day and inter-day precision were less than 6.77%, indicating good repeatability. The adsorbent showed promising prospects for the efficient enrichment of trace amounts of aflatoxins in complex food matrices.
Graphical Abstract




Similar content being viewed by others
References
Meggs WJ (2009) Epidemics of mold poisoning past and present. Toxicol Ind Health 25(9-10):571–576. https://doi.org/10.1177/0748233709348277
Iqbal SZ, Asi MR, Jinap S (2014) Aflatoxins in dates and dates products. Food Control 43:163–166. https://doi.org/10.1016/j.foodcont.2014.03.010
Zhuang Z, Huang Y, Yang Y, Wang S (2016) Identification of AFB1-interacting proteins and interactions between RPSA and AFB1. J Hazard Mater 301:297–303. https://doi.org/10.1016/j.jhazmat.2015.08.053
Mishra HN, Das C (2003) A review on biological control and metabolism of aflatoxin. Crit Rev Food Sci Nutr 43(3):245–264. https://doi.org/10.1080/10408690390826518
Li J, Xu X, Guo W, Zhang Y, Feng X, Zhang F (2022) Synthesis of a magnetic covalent organic framework as sorbents for solid-phase extraction of aflatoxins in food prior to quantification by liquid chromatography-mass spectrometry. Food Chem 387:132821. https://doi.org/10.1016/j.foodchem.2022.132821
Krska R, Schubert-Ullrich P, Molinelli A, Sulyok M, MacDonald S, Crews C (2008) Mycotoxin analysis: an update. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(2):152–163. https://doi.org/10.1080/02652030701765723
Jiménez Medina ML, Lafarga T, Garrido Frenich A, Romero-González R (2019) Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: a review (from 2010 to 2019). Food Rev Intl 37(3):244–275. https://doi.org/10.1080/87559129.2019.1701009
Maggira M, Ioannidou M, Sakaridis I, Samouris G (2021) Determination of aflatoxin M1 in raw milk using an HPLC-FL method in comparison with commercial ELISA kits-application in raw milk samples from various regions of Greece. Vet Sci 8(3). https://doi.org/10.3390/vetsci8030046
López Grío SJ, Garrido Frenich A, Martínez Vidal JL, Romero-González R (2010) Determination of aflatoxins B1, B2, G1, G2 and ochratoxin A in animal feed by ultra high-performance liquid chromatography-tandem mass spectrometry. J Sep Sci 33(4-5):502–508. https://doi.org/10.1002/jssc.200900663
Xie L, Chen M, Ying Y (2016) Development of methods for determination of aflatoxins. Crit Rev Food Sci Nutr 56(16):2642–2664. https://doi.org/10.1080/10408398.2014.907234
Parker WA, Melnick D (1966) Absence of aflatoxin from refined vegetable oils. J Am Oil Chem Soc 43(11):635–638. https://doi.org/10.1007/bf02640803
Zhou J, Xu JJ, Huang BF, Cai ZX, Ren YP (2017) High-performance liquid chromatographic determination of multi-mycotoxin in cereals and bean foodstuffs using interference-removal solid-phase extraction combined with optimized dispersive liquid-liquid microextraction. J Sep Sci 40(10):2141–2150. https://doi.org/10.1002/jssc.201601326
Ardic M, Karakaya Y, Atasever M, Durmaz H (2008) Determination of aflatoxin B(1) levels in deep-red ground pepper (isot) using immunoaffinity column combined with ELISA. Food Chem Toxicol 46(5):1596–1599. https://doi.org/10.1016/j.fct.2007.12.025
Frenich AG, Romero-González R, Gómez-Pérez ML, Vidal JL (2011) Multi-mycotoxin analysis in eggs using a QuEChERS-based extraction procedure and ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. J Chromatogr A 1218(28):4349–4356. https://doi.org/10.1016/j.chroma.2011.05.005
Arabi M, Ostovan A, Bagheri AR, Guo X, Wang L, Li J et al (2020) Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC Trends Anal Chem 128:115923. https://doi.org/10.1016/j.trac.2020.115923
Anklam E, Berg H, Mathiasson L, Sharman M, Ulberth F (1998) Supercritical fluid extraction (SFE) in food analysis: a review. Food Addit Contam 15(6):729–750. https://doi.org/10.1080/02652039809374703
Wu Y, Yu B, Cui P, Yu T, Shi G, Shen Z (2021) Development of a quantum dot-based lateral flow immunoassay with high reaction consistency to total aflatoxins in botanical materials. Anal Bioanal Chem 413(6):1629–1637. https://doi.org/10.1007/s00216-020-03123-4
Vojoudi H, Badiei A, Bahar S, Mohammadi Ziarani G, Faridbod F, Ganjali MR (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J Magn Magn Mater 441:193–203. https://doi.org/10.1016/j.jmmm.2017.05.065
Vojoudi H, Badiei A, Banaei A, Bahar S, Karimi S, Mohammadi Ziarani G et al (2017) Extraction of gold, palladium and silver ions using organically modified silica-coated magnetic nanoparticles and silica gel as a sorbent. Microchimica Acta 184(10):3859–3866. https://doi.org/10.1007/s00604-017-2414-x
Modheji M, Emadi H, Vojoudi H (2020) Efficient pre-concentration of As(III) in food samples using guanidine-modified magnetic mesoporous silica. J Porous Mater 27(4):971–978. https://doi.org/10.1007/s10934-020-00873-5
Banaei A, Vojoudi H, Karimi S, Bahar S, Pourbasheer E (2015) Synthesis and characterization of new modified silica coated magnetite nanoparticles with bisaldehyde as selective adsorbents of Ag(i) from aqueous samples. RSC Adv 5(101):83304–83313. https://doi.org/10.1039/c5ra11765h
Vojoudi H, Badiei A, Bahar S, Mohammadi Ziarani G, Faridbod F, Ganjali MR (2017) Post-modification of nanoporous silica type SBA-15 by bis(3-triethoxysilylpropyl)tetrasulfide as an efficient adsorbent for arsenic removal. Powder Technol 319:271–278. https://doi.org/10.1016/j.powtec.2017.06.028
Banaei A, Farokhi Yaychi M, Karimi S, Vojoudi H, Namazi H, Badiei A et al (2018) 2,2’-(butane-1,4-diylbis(oxy))dibenzaldehyde cross-linked magnetic chitosan nanoparticles as a new adsorbent for the removal of reactive red 239 from aqueous solutions. Mater Chem Phys 212:1–11. https://doi.org/10.1016/j.matchemphys.2018.02.036
Wang D, Jana D, Zhao Y (2020) Metal-organic framework derived nanozymes in biomedicine. Acc Chem Res 53(7):1389–1400. https://doi.org/10.1021/acs.accounts.0c00268
Li Z, Zhang Y, Xia H, Mu Y, Liu X (2016) A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu(2+) ions. Chem Commun (Camb) 52(39):6613–6616. https://doi.org/10.1039/c6cc01476c
Esrafili A, Wagner A, Inamdar S, Acharya AP (2021) Covalent organic frameworks for biomedical applications. Adv Healthc Mater 10(6):e2002090. https://doi.org/10.1002/adhm.202002090
Blesa J, Soriano JM, Moltó JC, Marín R, Mañes J (2003) Determination of aflatoxins in peanuts by matrix solid-phase dispersion and liquid chromatography. J Chromatogr A 1011(1-2):49–54. https://doi.org/10.1016/s0021-9673(03)01102-6
Chen L, Zhang M, Fu F, Li J, Lin Z (2018) Facile synthesis of magnetic covalent organic framework nanobeads and application to magnetic solid-phase extraction of trace estrogens from human urine. J Chromatogr A 1567:136–146. https://doi.org/10.1016/j.chroma.2018.06.066
Lin G, Gao C, Zheng Q, Lei Z, Geng H, Lin Z et al (2017) Room-temperature synthesis of core-shell structured magnetic covalent organic frameworks for efficient enrichment of peptides and simultaneous exclusion of proteins. Chem Commun (Camb) 53(26):3649–3652. https://doi.org/10.1039/c7cc00482f
Li CY, Liu JM, Wang ZH, Lv SW, Zhao N, Wang S (2020) Integration of Fe(3)O(4)@UiO-66-NH(2)@MON core-shell structured adsorbents for specific preconcentration and sensitive determination of aflatoxins against complex sample matrix. J Hazard Mater 384:121348. https://doi.org/10.1016/j.jhazmat.2019.121348
Dhanshetty M, Banerjee K (2019) Simultaneous direct analysis of aflatoxins and ochratoxin a in cereals and their processed products by ultra-high performance liquid chromatography with fluorescence detection. J AOAC Int 102(6):1666–1672. https://doi.org/10.5740/jaoacint.19-0048
Zhou NZ, Liu P, Su XC, Liao YH, Lei NS, Liang YH et al (2017) Low-cost humic acid-bonded silica as an effective solid-phase extraction sorbent for convenient determination of aflatoxins in edible oils. Anal Chim Acta 970:38–46. https://doi.org/10.1016/j.aca.2017.02.029
Pellicer-Castell E, Belenguer-Sapina C, Borras VJ, Amoros P, El Haskouri J, Herrero-Martinez JM et al (2019) Extraction of aflatoxins by using mesoporous silica (type UVM-7), and their quantitation by HPLC-MS. Mikrochim Acta 186(12):792. https://doi.org/10.1007/s00604-019-3958-8
Yu L, Ma F, Ding X, Wang H, Li P (2018) Silica/graphene oxide nanocomposites: Potential adsorbents for solid phase extraction of trace aflatoxins in cereal crops coupled with high performance liquid chromatography. Food Chem 245:1018–1024. https://doi.org/10.1016/j.foodchem.2017.11.070
Jayasinghe G, Dominguez-Gonzalez R, Bermejo-Barrera P, Moreda-Pineiro A (2020) Ultrasound assisted combined molecularly imprinted polymer for the selective micro-solid phase extraction and determination of aflatoxins in fish feed using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1609:460431. https://doi.org/10.1016/j.chroma.2019.460431
Zhao Y, Huang J, Ma L, Wang F (2017) Development and validation of a simple and fast method for simultaneous determination of aflatoxin B(1) and sterigmatocystin in grains. Food Chem 221:11–17. https://doi.org/10.1016/j.foodchem.2016.10.036
Jiménez Medina ML, Lafarga T, Garrido Frenich A, Romero-González R (2019) Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: a review (from 2010 to 2019). Food Rev Intl 37:244–275
Funding
This study was supported by the National Natural Science Foundation of China (32372430).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, D., Xu, X., Wang, X. et al. Synthesis of a core–shell magnetic covalent organic framework for the enrichment and detection of aflatoxin in food using HPLC-MS/MS. Microchim Acta 190, 488 (2023). https://doi.org/10.1007/s00604-023-06051-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06051-z