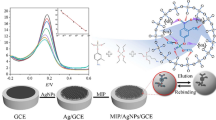
Abstract
A magnetic graphite-epoxy composite (m-GEC) electrochemical sensor is presented based on magnetic imprinted polymer (mag-MIP) to determine homocysteine (Hcy). Mag-MIP was synthesized via precipitation polymerization, using functionalized magnetic nanoparticles (Fe3O4) together with the template molecule (Hcy), the functional monomer 2-hydroxyethyl methacrylate (HEMA), and the structural monomer trimethylolpropane trimethacrylate (TRIM). For mag-NIP (magnetic non-imprinted polymer), the procedure was the same in the absence of Hcy. Morphological and structural properties of the resultant mag-MIP and mag-NIP were examined using TEM, FT-IR, and Vibrating Sample Magnetometer. Under optimized conditions, the m-GEC/mag-MIP sensor showed a linear range of 0.1–2 µmol L−1, with a limit of detection (LOD) of 0.030 µmol L−1. In addition, the proposed sensor responded selectively to Hcy compared to several interferents present in biological samples. The recovery values determined by differential pulse voltammetry (DPV) were close to 100% for natural and synthetic samples, indicating good method accuracy. The developed electrochemical sensor proves to be a suitable device for determining Hcy, with advantages related to magnetic separation and electrochemical analysis.
Highlights
• A novel mag-MIP with specific recognition sites for selective detection of homocysteine (Hcy) was prepared
• The prepared platform allowed successful recovery percentages of Hcy in different matrices
• Differential pulse voltammetry (DPV) was applied in urine and synthetic plasma
• Selectivity was obtained in the presence of other biothiols
• This platform showed advantages related to a combined magnetic separation and an electrochemical analysis
• The sustainability of the procedure concerns easier and cheaper pre-treatment and a biomimetic replacement of unstable biomolecules
AbstractSection Graphical Abstract






Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Bremner JD, Goldberg J, Vaccarino V (2021) Plasma homocysteine concentrations and depression: a twin study. J Affect Disord Reports 4:1–7. https://doi.org/10.1016/j.jadr.2021.100087
Sbodio JI, Snyder SH, Paul BD (2019) Regulators of the transsulfuration pathway. Br J Pharmacol 176:583–593. https://doi.org/10.1111/bph.14446
Aguilar B, Rojas JC, Collados MT (2004) Metabolism of homocysteine and its relationship with cardiovascular disease. J Thromb Thrombolysis 18:75–87. https://doi.org/10.1007/s11239-004-0204-x
Feng Y, Kang K, Xue Q et al (2020) Value of plasma homocysteine to predict stroke, cardiovascular diseases, and new-onset hypertension. Medicine (Baltimore) 99:1–10. https://doi.org/10.1097/MD.0000000000021541
Ogundeji SP, Kotila TR, Fasola FA (2020) Hyperhomocysteinemia is associated with deep vein thrombosis in Nigerian patients. Egypt J Haematol 45:111–114. https://doi.org/10.4103/ejh.ejh
Piazzolla G, Candigliota M, Fanelli M et al (2019) Hyperhomocysteinemia is an independent risk factor of atherosclerosis in patients with metabolic syndrome. Diabetol Metab Syndr 11:1–9. https://doi.org/10.1186/s13098-019-0484-0
Muzaffar R, Khan MA, Mushtaq MH et al (2023) Hyperhomocysteinemia as an independent risk factor for coronary reart disease. Comparison with conventional risk factors. Braz J Biol 83:1–8. https://doi.org/10.1590/1519-6984.249104
Poddar R (2021) Hyperhomocysteinemia is an emerging comorbidity in ischemic stroke. Exp Neurol 336:1–9. https://doi.org/10.1016/j.expneurol.2020.113541
Zaric BL, Obradovic M, Bajic V et al (2019) Homocysteine and hyperhomocysteinaemia. Curr Med Chem 26:2948–2961. https://doi.org/10.2174/0929867325666180313105949
Zhang D, Wen X, Wu W et al (2015) Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS One 10:1–16. https://doi.org/10.1371/journal.pone.0123423
Yang Q, He G-W (2019) Imbalance of homocysteine and H2S: significance, mechanisms, and therapeutic promise in vascular injury. Oxid Med Cell Longev 2019:1–11. https://doi.org/10.1155/2019/7629673
Rajaram R, Mathiyarasu J (2018) An electrochemical sensor for homocysteine detection using gold nanoparticle incorporated reduced graphene oxide. Colloids Surf B Biointerfaces 170:109–114. https://doi.org/10.1016/j.colsurfb.2018.05.066
Wen X, Zhao X, Peng B et al (2021) Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl Surf Sci 556:1–8. https://doi.org/10.1016/j.apsusc.2021.149735
Hosseinzadeh L, Khoshroo A, Adib K et al (2021) Determination of homocysteine using a dopamine-functionalized graphene composite. Microchem J 165:1–6. https://doi.org/10.1016/j.microc.2021.106124
Lee PT, Lowinsohn D, Compton RG (2014) The selective electrochemical detection of homocysteine in the presence of glutathione, cysteine, and ascorbic acid using carbon electrodes. Analyst 139:3755–3762. https://doi.org/10.1039/c4an00372a
Salehzadeh H, Mokhtari B, Nematollahi D (2014) Selective electrochemical determination of homocysteine in the presence of cysteine and glutathione. Electrochim Acta 123:353–361. https://doi.org/10.1016/j.electacta.2014.01.072
Agüí L, Peña-Farfal C, Yáñez-Sedeño P, Pingarrón JM (2007) Electrochemical determination of homocysteine at a gold nanoparticle-modified electrode. Talanta 74:412–420. https://doi.org/10.1016/j.talanta.2007.05.035
Lawrence NS, Deo RP, Wang J (2004) Detection of homocysteine at carbon nanotube paste electrodes. Talanta 63:443–449. https://doi.org/10.1016/j.talanta.2003.11.024
Gong K, Dong Y, Xiong S et al (2004) Novel electrochemical method for sensitive determination of homocysteine with carbon nanotube-based electrodes. Biosens Bioelectron 20:253–259. https://doi.org/10.1016/j.bios.2004.01.014
Kuhn J, Aylaz G, Sari E et al (2020) Selective binding of antibiotics using magnetic molecular imprint polymer (MMIP) networks prepared from vinyl-functionalized magnetic nanoparticles. J Hazard Mater 387:1–10. https://doi.org/10.1016/j.jhazmat.2019.121709
Khan S, Wong A, Valnice M et al (2019) Electrochemical sensors based on biomimetic magnetic molecularly imprinted polymer for selective quantification of methyl green in environmental samples. Mater Sci Eng C 103:1–7. https://doi.org/10.1016/j.msec.2019.109825
Amatatongchai M, Sroysee W, Sodkrathok P et al (2019) Novel three-dimensional molecularly imprinted polymer-coated carbon nanotubes (3D-CNTs@MIP) for selective detection of profenofos in food. Anal Chim Acta 1076:64–72. https://doi.org/10.1016/j.aca.2019.04.075
Yao Z, Yang X, Liu X et al (2018) Electrochemical quercetin sensor based on a nanocomposite consisting of magnetized reduced graphene oxide, silver nanoparticles and a molecularly imprinted polymer on a screen-printed electrode. Microchim Acta 185:1–9. https://doi.org/10.1007/s00604-017-2613-5
Yáñez-Sedeño P, Campuzano S, Pingarrón JM (2017) Electrochemical sensors based on magnetic molecularly imprinted polymers: a review. Anal Chim Acta 960:1–17. https://doi.org/10.1016/j.aca.2017.01.003
Madhavi J, Prasad V, Reddy KR et al (2021) Facile synthesis of Ni-doped ZnS-CdS composite and their magnetic and photoluminescence properties. J Environ Chem Eng 9:1–8. https://doi.org/10.1016/j.jece.2021.106335
Sateesha KM, Pasha M, Pati M et al (2022) Syntheses and magnetic properties of substituted bis-indacenyl bi-metallic complexes & application. Rev Colomb Quim 51:58–64. https://doi.org/10.15446/rev.colomb.quim.v51n1.101889
Dinc M, Esen C, Mizaikoff B (2019) Recent advances on core e shell magnetic molecularly imprinted polymers for biomacromolecules. Trends Anal Chem 114:202–217. https://doi.org/10.1016/j.trac.2019.03.008
Bergamin B, Pupin RR, Wong A, Sotomayor MDPT (2019) A new electrochemical platform based on a polyurethane composite electrode modified with magnetic nanoparticles coated with molecularly imprinted polymer for the determination of estradiol valerate in different matrices. J Braz Chem Soc 30:2344–2354. https://doi.org/10.21577/0103-5053.20190142
Kong X, Li F, Li Y et al (2020) Molecularly imprinted polymer functionalized magnetic Fe3O4 for the highly selective extraction of triclosan. J Sep Sci 43:808–817. https://doi.org/10.1002/jssc.201900924
Wong A, Foguel MV, Khan S et al (2015) Development of an electrochemical sensor modified with MWCNT-COOH and Mip for detection of diuron. Electrochim Acta 182:122–130. https://doi.org/10.1016/j.electacta.2015.09.054
Kong X, Gao R, He X et al (2012) Synthesis and characterization of the core-shell magnetic molecularly imprinted polymers (Fe3O4@MIPs) adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J Chromatogr A 1245:8–16. https://doi.org/10.1016/j.chroma.2012.04.061
Huynh T-P, Le T (2018) Synthetic chemistry for molecular imprinting. In: Kutner W, Sharma PS (eds) Molecularly imprinted polymers for analytical chemistry applications, 1st edn. Royal Society of Chemistry, Cambridge, pp 28–64
Pividori MI, Alegret S (2003) Graphite-epoxy platforms for electrochemical genosensing. Anal Lett 36:1669–1695. https://doi.org/10.1081/AL-120023607
Laube N, Mohr B, Hesse A (2001) Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J Cryst Growth 233:367–374. https://doi.org/10.1016/S0022-0248(01)01547-0
Liu L, Qiu CL, Chen Q, Zhang SM (2006) Corrosion behavior of Zr-based bulk metallic glasses in different artificial body fluids. J Alloys Compd 425:268–273. https://doi.org/10.1016/j.jallcom.2006.01.048
Santos ACF, de Araújo ORP, Moura FA et al (2021) Development of magnetic nanoparticles modified with new molecularly imprinted polymer (MIPs) for selective analysis of glutathione. Sensors Actuators B Chem 344:1–10. https://doi.org/10.1016/j.snb.2021.130171
Soltani H, Beitollahi H, Hatefi-Mehrjardi A-H et al (2014) Nanostructured base electrochemical sensor for voltammetric determination of homocysteine using a modified single-walled carbon nanotubes paste electrode. Ionics (Kiel) 20:1481–1488. https://doi.org/10.1007/s11581-014-1099-y
Acknowledgements
The authors wish to thank Prof. Maria Isabel Pividori (Universitat Autònoma de Barcelona) for the kind donation of the electrodes m-GEC.
Funding
The authors are grateful for the financial support provided by the Brazilian research funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process 435704/2018–4) and fellowships (MOFG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brazil (CAPES)—Finance Code 001, CAPES (88882.316125/2019–01), CAPES/RENORBIO/PROAP (23038.011373/2017–31), INCT-Bioanalítica (process 465389/2014–7), Fundação de Apoio à Pesquisa de Alagoas (FAPEAL), Programa de Pós graduação em Química e Biotecnologia, and RENORBIO.
Author information
Authors and Affiliations
Contributions
Poliana da Conceição: Methodology, Validation, Investigation. Antonio Gomes dos Santos Neto: Investigation, Methodology. Ana Caroline Ferreira Santos: Methodology, Validation, Investigation, Writing—original draft. Sabir Khan: Investigation, Methodology, Formal analysis, Writing- review & editing, Writing—original draft. Auro A. Tanaka: Data curation, Methodology, Formal analysis, Writing- review & editing. Antonio Euzébio Goulart Santana: Investigation, Supervision, Review & editing original draft. Maria Del Pilar Taboada Sotomayor: Investigation, Validation, Writing—Review & Editing, Writing—Original draft. Marília O.F.Goulart: Conceptualization, Supervision, Project administration, Funding acquisition, Writing—review & editing, Writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Conceição, P., dos Santos Neto, A.G., Khan, S. et al. Extraction-assisted voltammetric determination of homocysteine using magnetic nanoparticles modified with molecularly imprinted polymer. Microchim Acta 190, 159 (2023). https://doi.org/10.1007/s00604-023-05738-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05738-7