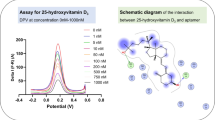
Abstract
A sandwich-type electrochemical aptasensor was designed for sensitive detection of leptin in biological samples, including human serum and human plasma. The developed aptasensor was produced by electrodeposition of gold nanoparticles on a screen-printed electrode modified with zinc oxide nanoparticles. The synergy effect of zinc oxide and gold nanoparticles improved the electrocatalytic activity of the aptasensor. The obtained high surface area allowed more aptamer molecules to be loaded on the electrode surface. Signal amplification significantly increases the detection sensitivity of a developed biosensor. Although the use of nanomaterials is the most preferred detection tool for this purpose, as an alternative, enzyme-catalyzed signal amplification is widely used in the construction of a biosensor due to its specificity and high catalytic efficiency. Therefore, both nanomaterial-supported and an alkaline phosphatase-based aptasensor design were developed, which can produce in situ electroactive product by enzymatic hydrolysis of the inactive substrate to achieve a higher signal-to-background ratio. Under optimal conditions, the developed aptasensor exhibited a wide linear concentration range from 0.01 pg mL−1 to 100.0 pg mL−1 with a detection limit of 0.0035 pg mL−1. While the developed aptasensor provided excellent selectivity in the presence of some interfering compounds, it possessed outstanding reproducibility and stability. In addition, the developed aptasensor has been applied with good recoveries in the range 96.31 to 108.79% in human serum and plasma samples. In conclusion, all the obtained results showed the feasibility of the developed aptasensor for practical applications.
Graphical abstract









Similar content being viewed by others
References
McAllister TW (2011) Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 13. https://doi.org/10.31887/dcns.2011.13.2/tmcallister
Bouchard HC, Sun D, Dennis EL et al (2022) Age-dependent white matter disruptions after military traumatic brain injury: multivariate analysis results from ENIGMA brain injury. Hum Brain Mapp. https://doi.org/10.1002/hbm.25811
Mollayeva T, Mollayeva S, Colantonio A (2018) Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol 14(12):711–722. https://doi.org/10.1038/s41582-018-0091-y
Daoud H, Alharfi I, Alhelali I et al (2014) Brain injury biomarkers as outcome predictors in pediatric severe traumatic brain injury. Neurocrit Care 20. https://doi.org/10.1007/s12028-013-9879-1
Jiang W, Zou W, Hu M, et al (2022) Hydrogen sulphide attenuates neuronal apoptosis of substantia nigra by re-establishing autophagic flux via promoting leptin signalling in a 6-hydroxydopamine rat model of Parkinson’s disease. Clin Exp Pharmacol Physiol 49. https://doi.org/10.1111/1440-1681.13587
Toklu HZ, Yang Z, Oktay S, et al (2018) Overpressure blast injury-induced oxidative stress and neuroinflammation response in rat frontal cortex and cerebellum. Behav Brain Res 340. https://doi.org/10.1016/j.bbr.2017.04.025
Wang S, Yan X, Yang Y et al (2021) Advances and perspectives of aptasensors for the detection of tetracyclines: a class of model compounds of food analysis. Food Chem 364:130361. https://doi.org/10.1016/j.foodchem.2021.130361
Zhang N, Li J, Liu B et al (2022) Signal enhancing strategies in aptasensors for the detection of small molecular contaminants by nanomaterials and nucleic acid amplification. Talanta 236:122866. https://doi.org/10.1016/j.talanta.2021.122866
Melinte G, Selvolini G, Cristea C, Marrazza G (2021) Aptasensors for lysozyme detection: Recent advances. Talanta 226:122169. https://doi.org/10.1016/j.talanta.2021.122169
Bozal-Palabiyik B, Uslu B, Marrazza G (2019) Nanosensors in biomarker detection. In: New Developments in nanosensors for pharmaceutical analysis. https://doi.org/10.1016/B978-0-12-816144-9.00011-0
He B, Dong X (2019) Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sensors Actuators, B Chem 294. https://doi.org/10.1016/j.snb.2019.05.045
Yang SL, Li G, Feng J, et al (2022) Synthesis of core/satellite donut-shaped ZnO–Au nanoparticles incorporated with reduced graphene oxide for electrochemical sensing of rutin. Electrochim Acta 412. https://doi.org/10.1016/j.electacta.2022.140157
Kenarkob M, Pourghobadi Z (2019) Electrochemical sensor for acetaminophen based on a glassy carbon electrode modified with ZnO/Au nanoparticles on functionalized multi-walled carbon nano-tubes. Microchem J 146. https://doi.org/10.1016/j.microc.2019.02.038
Rovina K, Siddiquee S (2016) Electrochemical sensor based rapid determination of melamine using ionic liquid/zinc oxide nanoparticles/chitosan/gold electrode. Food Control 59. https://doi.org/10.1016/j.foodcont.2015.07.009
Ezhil Vilian AT, Kang SM, Yeong Oh S et al (2020) A simple strategy for the synthesis of flower-like textures of Au-ZnO anchored carbon nanocomposite towards the high‐performance electrochemical sensing of sunset yellow. Food Chem 323. https://doi.org/10.1016/j.foodchem.2020.126848
Fang L, Huang K, Zhang B, et al (2014) Nanosheet-based 3D hierarchical ZnO structure decorated with Au nanoparticles for enhanced electrochemical detection of dopamine. RSC Adv 4. https://doi.org/10.1039/c4ra06090c
Madhu R, Dinesh B, Chen SM et al (2015) An electrochemical synthesis strategy for composite based ZnO microspheres-Au nanoparticles on reduced graphene oxide for the sensitive detection of hydrazine in water samples. RSC Adv 5. https://doi.org/10.1039/c5ra05612h
Wang M, Fu Z, Li B, et al (2014) One-step, ultrasensitive, and electrochemical assay of microRNAs based on t7 exonuclease assisted cyclic enzymatic amplification. Anal Chem 86. https://doi.org/10.1021/ac5010376
Wen Y, Pei H, Shen Y, et al (2012) DNA Nanostructure-based ınterfacial engineering for PCR-free ultrasensitive electrochemical analysis of microRNA. Sci Rep 2. https://doi.org/10.1038/srep00867
Yang H (2012) Enzyme-based ultrasensitive electrochemical biosensors. Curr Opin Chem Biol 16(3–4):422–428. https://doi.org/10.1016/j.cbpa.2012.03.015
Melinte G, Hosu O, Ștefan G, et al (2022) Poly-L-Lysine@gold nanostructured hybrid platform for Lysozyme aptamer sandwich-based detection. Electrochim Acta 403. https://doi.org/10.1016/j.electacta.2021.139718
Ashley J, Li SFY (2013) Three-dimensional selection of leptin aptamers using capillary electrophoresis and implications for clone validation. Anal Biochem 434:146–152. https://doi.org/10.1016/j.ab.2012.11.024
ERKMEN C, TIĞ GA, USLU B (2022) First label-free impedimetric aptasensor based on Au NPs/TiO2 NPs for the determination of leptin. Sensors Actuators B Chem 358. https://doi.org/10.1016/j.snb.2022.131420
Rasheed PA, Sandhyarani N (2017) Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta 184:981–1000. https://doi.org/10.1007/s00604-017-2143-1
Srujana S, Bhagat D (2022) Chemical-based synthesis of ZnO nanoparticles and their applications in agriculture. Nanotechnol Environ Eng 7. https://doi.org/10.1007/s41204-022-00224-6
Prabhu K, Malode SJ, Shetti NP, Kulkarni RM (2022) Analysis of herbicide and its applications through a sensitive electrochemical technique based on MWCNTs/ZnO/CPE fabricated sensor. Chemosphere 287. https://doi.org/10.1016/j.chemosphere.2021.132086
Svigelj R, Zuliani I, Grazioli C et al (2022) An effective label-free electrochemical aptasensor based on gold nanoparticles for gluten detection. Nanomaterials 12. https://doi.org/10.3390/nano12060987
Chen H, Wang Y, Li X, et al (2018) A CO2-tunable plasmonic nanosensor based on the interfacial assembly of gold nanoparticles on diblock copolymers grafted from gold surfaces. RSC Adv 8. https://doi.org/10.1039/c8ra02934b
Jafari S, Dehghani M, Nasirizadeh N, et al (2019) Label-free electrochemical detection of Cloxacillin antibiotic in milk samples based on molecularly imprinted polymer and graphene oxide-gold nanocomposite. Meas J Int Meas Confed 145. https://doi.org/10.1016/j.measurement.2019.05.068
Srivastava M, Nirala NR, Srivastava SK, Prakash R (2018) A comparative study of aptasensor vs ımmunosensor for label-free PSA Cancer detection on GQDs-AuNRs modified screen-printed electrodes. Sci Rep 8. https://doi.org/10.1038/s41598-018-19733-z
Nazari M, Kashanian S, Rafipour R, Omidfar K (2019) Biosensor design using an electroactive label-based aptamer to detect bisphenol A in serum samples. J Biosci 44. https://doi.org/10.1007/s12038-019-9921-3
Nia NG, Azadbakht A (2018) Nanostructured aptamer-based sensing platform for highly sensitive recognition of myoglobin. Microchim Acta 185. https://doi.org/10.1007/s00604-018-2860-0
Muhammad A, Yusof NA, Hajian R, Abdullah J (2016) Construction of an electrochemical sensor based on carbon nanotubes/gold nanoparticles for trace determination of amoxicillin in bovine milk. Sensors (Switzerland) 16. https://doi.org/10.3390/s16010056
Saeb E, Asadpour-Zeynali K (2021) Facile synthesis of TiO2@PANI@Au nanocomposite as an electrochemical sensor for determination of hydrazine. Microchem J 160:105603. https://doi.org/10.1016/j.microc.2020.105603
Salih E, Mekawy M, Hassan RYA, El-Sherbiny IM (2016) Synthesis, characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J Nanostructure Chem 6. https://doi.org/10.1007/s40097-016-0188-z
Heydari M, Ghoreishi SM, Khoobi A (2019) Chemometrics-assisted determination of Sudan dyes using zinc oxide nanoparticle-based electrochemical sensor. Food Chem 283. https://doi.org/10.1016/j.foodchem.2018.12.132
Kurbanoglu S, Ozkan SA (2018) Electrochemical carbon based nanosensors: a promising tool in pharmaceutical and biomedical analysis. J Pharm Biomed Anal 147:439–457. https://doi.org/10.1016/j.jpba.2017.06.062
García-González R, Fernández Abedul MT (2020) Electrochemical impedance spectroscopy for characterization of electrode surfaces. In: Laboratory methods in dynamic electroanalysis. https://doi.org/10.1016/B978-0-12-815932-3.00012-7
Nodehi M, Baghayeri M, Behazin R, Veisi H (2021) Electrochemical aptasensor of bisphenol A constructed based on 3D mesoporous structural SBA-15-Met with a thin layer of gold nanoparticles. Microchem J 162. https://doi.org/10.1016/j.microc.2020.105825
Guo S, Wang E (2007) Synthesis and electrochemical applications of gold nanoparticles. Anal Chim Acta 598(2):181–192. https://doi.org/10.1016/j.aca.2007.07.054
Shi L, Wang Z, Bai L, Yang G (2022) Preparation of 3d nanoflower-like ZnO/graphene oxide decorated with Au@AuPt Bimetallic nanoparticles for electrochemical determination of doxorubicin hydrochloride. Int J Electrochem Sci 17. https://doi.org/10.20964/2022.1.45
Li F, Gao X, Wang X, et al (2022) Ultrasensitive sandwich RNA-aptasensor based on dual-signal amplification strategy for highly sensitive neomycin detection. Food Control 131. https://doi.org/10.1016/j.foodcont.2021.108445
Wang Y, Wang Y, Wang F et al (2022) Electrochemical aptasensor based on gold modified thiol graphene as sensing platform and gold-palladium modified zirconium metal-organic frameworks nanozyme as signal enhancer for ultrasensitive detection of mercury ions. J Colloid Interface Sci 606. https://doi.org/10.1016/j.jcis.2021.08.055
Liu J, Zhu B, Dong H et al (2022) A novel electrochemical insulin aptasensor: from glassy carbon electrodes to disposable, single-use laser-scribed graphene electrodes. Bioelectrochemistry 143. https://doi.org/10.1016/j.bioelechem.2021.107995
Martins TS, Bott-Neto JL, Oliveira ON, Machado SAS (2022) A sandwich-type electrochemical immunosensor based on Au-rGO composite for CA15-3 tumor marker detection. Microchim Acta 189. https://doi.org/10.1007/s00604-021-05145-w
Gasparotto G, Costa JPC, Costa PI et al (2017) Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection. Mater Sci Eng C 76:1240–1247. https://doi.org/10.1016/j.msec.2017.02.031
Gholizadeh A, Shahrokhian S, Iraji Zad A, et al (2012) Fabrication of sensitive glutamate biosensor based on vertically aligned CNT nanoelectrode array and investigating the effect of CNTs density on the electrode performance. Anal Chem 84. https://doi.org/10.1021/ac300463x
Zhou Y, Liu J, Dong H, et al (2022) Target-induced silver nanocluster generation for highly sensitive electrochemical aptasensor towards cell-secreted interferon-γ. Biosens Bioelectron 203. https://doi.org/10.1016/j.bios.2022.114042
Mihailescu CM, Stan D, Savin M et al (2020) Platform with biomimetic electrochemical sensors for adiponectin and leptin detection in human serum. Talanta 210:120643. https://doi.org/10.1016/j.talanta.2019.120643
Chen W, Lei Y, Li CM (2010) Regenerable leptin immunosensor based on protein G immobilized au-pyrrole propylic acid-polypyrrole nanocomposite. Electroanalysis 22:1078–1083. https://doi.org/10.1002/elan.200900536
Dong F, Luo R, Chen H et al (2014) Amperometric immunosensor based on carbon nanotubes/chitosan film modified electrodes for detection of human leptin. Int J Electrochem Sci 9:6924–6935
He Y, Sun J, Wang X, Wang L (2015) Detection of human leptin in serum using chemiluminescence immunosensor: signal amplification by hemin/G-quadruplex DNAzymes and protein carriers by Fe3O4/polydopamine/Au nanocomposites. Sensors Actuators B Chem 221:792–798. https://doi.org/10.1016/j.snb.2015.07.022
Cai J, Gou X, Sun B et al (2019) Porous graphene-black phosphorus nanocomposite modified electrode for detection of leptin. Biosens Bioelectron 137:88–95. https://doi.org/10.1016/j.bios.2019.04.045
Zhang Q, Qing Y, Huang X et al (2018) Synthesis of single-walled carbon nanotubes–chitosan nanocomposites for the development of an electrochemical biosensor for serum leptin detection. Mater Lett 211:348–351. https://doi.org/10.1016/j.matlet.2017.10.036
Ojeda I, Moreno-Guzmán M, González-Cortés A et al (2013) A disposable electrochemical immunosensor for the determination of leptin in serum and breast milk. Analyst 138:4284–4291. https://doi.org/10.1039/c3an00183k
Liu X, Tseng CL, Lin LY et al (2021) Template-free synthesis of mesoporous Ce3NbO7/CeO2 hollow nanospheres for label-free electrochemical immunosensing of leptin. Sensors Actuators, B Chem 341:130005. https://doi.org/10.1016/j.snb.2021.130005
Özcan B, Sezgintürk MK (2021) A novel and disposable GP- based impedimetric biosensor using electropolymerization process with PGA for highly sensitive determination of leptin: Early diagnosis of childhood obesity. Talanta 225. https://doi.org/10.1016/j.talanta.2020.121985
Sung R, Seok Heo Y (2021) Sandwich elisa-based electrochemical biosensor for leptin in control and diet-induced obesity mouse model. Biosensors 11:1–9. https://doi.org/10.3390/bios11010007
Cavallo FR, Mirza KB, De Mateo S et al (2021) Aptasensor for quantification of leptin through pCR amplification of short DNA-aptamers. ACS Sensors 6:709–715. https://doi.org/10.1021/acssensors.0c02605
He Y, Wang X, Zhang Y et al (2013) An ultrasensitive chemiluminescent immunosensor for the detection of human leptin using hemin/G-quadruplex DNAzymes-assembled signal amplifier. Talanta 116:816–821. https://doi.org/10.1016/j.talanta.2013.07.074
Du Q, Yang DB, Shen YF, et al (2013) Plasma leptin level predicts hematoma growth and early neurological deterioration after acute intracerebral hemorrhage. Peptides 45. https://doi.org/10.1016/j.peptides.2013.04.017
Lin C, Huang SJ, Wang N, Shen ZP (2012) Relationship between plasma leptin levels and clinical outcomes of pediatric traumatic brain injury. Peptides 35:166–171. https://doi.org/10.1016/j.peptides.2012.03.024
Acknowledgements
This work was produced from the PhD thesis of Cem Erkmen (Ankara University, Health Sciences Institute). The Council of Higher Education (YOK) was greatly appreciated for providing scholarships under the special 100/2000 scholarship program to Cem Erkmen. Cem Erkmen also thanks to the financial support from The Scientific and Technological Research Council of Türkiye (TUBITAK) under the BIDEB/2211-A doctoral scholarship program.The authors acknowledge The Scientific and Technological Research Council of Türkiye (TUBITAK, project no.: 122Z695) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erkmen, C., Tığ, G.A. & Uslu, B. Nanomaterial-based sandwich-type electrochemical aptasensor platform for sensitive voltammetric determination of leptin. Microchim Acta 189, 396 (2022). https://doi.org/10.1007/s00604-022-05487-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05487-z