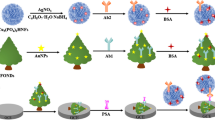
Abstract
Horseradish peroxidase (HRP) was highly loaded into large holes of nanometer-scale metal–organic frameworks (i.e., PCN-333(Al)) for signal amplification in enzyme-linked immunosorbent assay (ELISA). The enzyme-labeled antibody complex prepared using nanometer-scale PCN-333(Al) maintained a high catalytic efficiency. Its Vm and Kcat values with 3,3',5,5'-Tetramethylbenzidine (TMB)-H2O2 as substrates were 4.84 × 10−5 mM/s and 4.84 × 104 min−1, respectively. We demonstrated an HRP@PCN-333 signal amplification strategy for colorimetric assay of human prostate-specific antigen (PSA). The linear range of PSA detection by using this method was 15–165 pg/mL, and the limit of detection was 6 pg/mL (S/N = 3), indicating the potential application of this method in detecting disease markers under clinical conditions. The presented strategy exhibited the characteristics of significantly increased amount of labeled enzymes, improved stability and utilization of enzymes, simple preparation process of enzyme-labeled antibodies, and low cost.
Graphical abstract





taken from the ELISA of PSA at different standard concentrations, (B) corresponding calibration curve (■), and variation profile (□) of the detection results. (C) Calibration curve of PSA detected using an ELISA kit
Similar content being viewed by others
References
Kraaijeveld CA, Reed SE, Macnaughton MR (1980) Enzyme-linked immunosorbent assay for detection of antibody in volunteers experimentally infected with human coronavirus strain 229 E. J Clin Microbiol 12:493–497. https://doi.org/10.1128/jcm.12.4.493-497.1980
Chen Z, Wang H, Zhang Z, Chen L (2019) Chemical Redox-Cycling for Improving the Sensitivity of Colorimetric Enzyme-Linked Immunosorbent Assay. Anal Chem 91:1254–1259. https://doi.org/10.1021/acs.analchem.8b05095
Laing S, Hernandez-Santana A, Sassmannshausen J, Asquith DL, McInnes IB, Faulds K, Graham D (2011) Quantitative detection of human tumor necrosis factor alpha by a resonance raman enzyme-linked immunosorbent assay. Anal Chem 83:297–302. https://doi.org/10.1021/ac1024039
Gao L, Yang Q, Wu P, Li F (2020) Recent advances in nanomaterial-enhanced enzyme-linked immunosorbent assays. Analyst 145:4069–4078. https://doi.org/10.1039/D0AN00597E
Farka Z, Mickert MJ, Pastucha M, Mikusova Z, Skladal P, Gorris HH (2020) Advances in Optical Single-Molecule Detection: En Route to Supersensitive Bioaffinity Assays. Angew Chem Int Ed Engl 59:10746–10773. https://doi.org/10.1002/anie.201913924
Li D, Cui Y, Morisseau C, Wagner KM, Cho YS, Hammock BD (2020) Development of a Highly Sensitive Enzyme-Linked Immunosorbent Assay for Mouse Soluble Epoxide Hydrolase Detection by Combining a Polyclonal Capture Antibody with a Nanobody Tracer. Anal Chem 92:11654–11663. https://doi.org/10.1021/acs.analchem.0c01511
Huang J, Jiao L, Xu W, Fang Q, Wang H, Cai X, Yan H, Gu W, Zhu C (2021) Immobilizing Enzymes on Noble Metal Hydrogel Nanozymes with Synergistically Enhanced Peroxidase Activity for Ultrasensitive Immunoassays by Cascade Signal Amplification. ACS Appl Mater Interfaces 13:33383–33391. https://doi.org/10.1021/acsami.1c09100
Chen Y, Li P, Modica JA, Drout RJ, Farha OK (2018) Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J Am Chem Soc 140:5678–5681. https://doi.org/10.1021/jacs.8b02089
Li Q, Pan Y, Li H, Alhalhooly L, Li Y, Chen B, Choi Y, Yang Z (2020) Size-Tunable Metal-Organic Framework-Coated Magnetic Nanoparticles for Enzyme Encapsulation and Large-Substrate Biocatalysis. ACS Appl Mater Interfaces 12:41794–41801. https://doi.org/10.1021/acsami.0c13148
Balamurugan V, Varghese B, SowjanyaKumari S, Vinod Kumar K, Muthuchelvan D, Nagalingam M, Hemadri D, Roy P, Shome BR (2021) Avidin-Biotin recombinant nucleoprotein competitive ELISA for the detection of peste des petits ruminants virus antibodies in sheep and goats. J Virol Methods 295:114213. https://doi.org/10.1016/j.jviromet.2021.114213
Li J, Liu F, Zhu Z, Liu D, Chen X, Song Y, Zhou L, Yang C (2018) In Situ Pt Staining Method for Simple, Stable, and Sensitive Pressure-Based Bioassays. ACS Appl Mater Interfaces 10:13390–13396. https://doi.org/10.1021/acsami.8b03567
Chen C, Zhao D, Wang B, Ni P, Jiang Y, Zhang C, Yang F, Lu Y, Sun J (2020) Alkaline Phosphatase-Triggered in Situ Formation of Silicon-Containing Nanoparticles for a Fluorometric and Colorimetric Dual-Channel Immunoassay. Anal Chem 92:4639–4646. https://doi.org/10.1021/acs.analchem.0c00224
Gao Z, Lv S, Xu M, Tang D (2017) High-index hk0 faceted platinum concave nanocubes with enhanced peroxidase-like activity for an ultrasensitive colorimetric immunoassay of the human prostate-specific antigen. Analyst 142:911–917. https://doi.org/10.1039/C6AN02722A
Guo L, Xu S, Ma X, Qiu B, Lin Z, Chen G (2016) Dual-color plasmonic enzyme-linked immunosorbent assay based on enzyme-mediated etching of Au nanoparticles. Sci Rep 6:32755. https://doi.org/10.1038/srep32755
Ma L, Abugalyon Y, Li X (2021) Multicolorimetric ELISA biosensors on a paper/polymer hybrid analytical device for visual point-of-care detection of infection diseases. Anal Bioanal Chem 413:4655–4663. https://doi.org/10.1007/s00216-021-03359-8
Kaware M, Bronshtein A, Safi J, Van Emon JM, Chuang JC, Hock B, Kramer K, Altstein M (2006) Enzyme-linked immunosorbent assay (ELISA) and sol-gel-based immunoaffinity purification (IAP) of the pyrethroid bioallethrin in food and environmental samples. J Agric Food Chem 54:6482–6492. https://doi.org/10.1021/jf0607415
Delgado M, Lee KJ, Altobell L 3rd, Spanka C, Wentworth P Jr, Janda KD (2002) A parallel approach to the discovery of carrier delivery vehicles to enhance antigen immunogenicity. J Am Chem Soc 124:4946–4947. https://doi.org/10.1021/ja025715b
Zhang Y, Yang J, Nie J, Yang J, Gao D, Zhang L, Li J (2016) Enhanced ELISA using a handheld pH meter and enzyme-coated microparticles for the portable, sensitive detection of proteins. Chem Commun (Camb) 52:3474–3477. https://doi.org/10.1039/C5CC09852A
Neumann MM, Volodkin D (2020) Porous antibody-containing protein microparticles as novel carriers for ELISA. Analyst 145:1202–1206. https://doi.org/10.1039/C9AN01888C
Wei T, Du D, Zhu MJ, Lin Y, Dai Z (2016) An Improved Ultrasensitive Enzyme-Linked Immunosorbent Assay Using Hydrangea-Like Antibody-Enzyme-Inorganic Three-in-One Nanocomposites. ACS Appl Mater Interfaces 8:6329–6335. https://doi.org/10.1021/acsami.5b11834
Garg M, Sharma AL, Singh S (2021) Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019–2020. Biosens Bioelectron 171:112703. https://doi.org/10.1016/j.bios.2020.112703
Cai G, Yan P, Zhang L, Zhou HC, Jiang HL (2021) Metal-Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem Rev. https://doi.org/10.1021/acs.chemrev.1c00243
Freund R, Zaremba O, Arnauts G, Ameloot R, Skorupskii G, Dinca M, Bavykina A, Gascon J, Ejsmont A, Goscianska J, Kalmutzki M, Lachelt U, Ploetz E, Diercks C, Wuttke S (2021) The Current Status of MOF and COF Applications. Angew Chem Int Ed Engl. https://doi.org/10.1002/anie.202106259
Feng D, Liu TF, Su J, Mathieu B, Wei Z, Wan W, Yuan D, Chen Y, Wang X, Wang K, Lian X, Gu Z, Jihye P, Zou X, Zhou HC (2015) Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat Commun 6:5979. https://doi.org/10.1038/ncomms6979
Park J, Feng D, Zhou HC (2015) Dual Exchange in PCN-333: A Facile Strategy to Chemically Robust Mesoporous Chromium Metal-Organic Framework with Functional Groups. J Am Chem Soc 137:11801–11809. https://doi.org/10.1021/jacs.5b07373
Zhao M, Li Y, Ma X, Xia M, Zhang Y (2019) Adsorption of cholesterol oxidase and entrapment of horseradish peroxidase in metal-organic frameworks for the colorimetric biosensing of cholesterol. Talanta 200:293–299. https://doi.org/10.1016/j.talanta.2019.03.060
Lian X, Erazo-Oliveras A, Pellois JP, Zhou HC (2017) High efficiency and long-term intracellular activity of an enzymatic nanofactory based on metal-organic frameworks. Nat Commun 8:2075. https://doi.org/10.1038/s41467-017-02103-0
Berglund G, Carlsson G, Smith A, Szöke H, Henriksen A, Hajdu J (2002) The catalytic pathway of horseradish peroxidase at high resolution. Nature 417:463–468. https://doi.org/10.1038/417463a
Gajhede M, Schuller D, Henriksen A, Smith AT, Poulos TL (1997) Crystal structure of horseradish peroxidase C at 2.15 A resolution. Nat Struct Mol Biol 4:1032–1038. https://doi.org/10.1038/nsb1297-1032
Cai X, Xie Z, Li D, Meruyert K, Zang S, Jiang H (2020) Nano-sized metal-organic frameworks: Synthesis and applications. Coord Chem Rev 417:213366. https://doi.org/10.1016/j.ccr.2020.213366
Melinda S, Nobuhiro Y, Jee A-Y, Steve G (2014) Colloidal-Sized Metal-Organic Frameworks: Synthesis and Applications. Acc Chem Res 47:459–469. https://doi.org/10.1021/ar400151n
Nguyen TK, Thanh NM, Mahiddine S (2014) Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem Rev 114:7610–7630. https://doi.org/10.1021/cr400544s
Feng D, Chung WC, Wei Z, Gu ZY, Jiang HL, Chen YP, Darensbourg DJ, Zhou HC (2013) Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination. J Am Chem Soc 135:17105–17110. https://doi.org/10.1021/ja408084j
Bosch M, Yuan S, Rutledge W, Zhou HC (2017) Stepwise Synthesis of Metal-Organic Frameworks. Acc Chem Res 50:857–865. https://doi.org/10.1021/acs.accounts.6b00457
Wang XG, Cheng Q, Yu Y, Zhang XZ (2018) Controlled Nucleation and Controlled Growth for Size Predicable Synthesis of Nanoscale Metal-Organic Frameworks (MOFs): A General and Scalable Approach. Angew Chem Int Ed Engl 57:7836–7840. https://doi.org/10.1002/anie.201803766
Xia X, Zeng J, Kyle Oetjen L, Li Q, Xia Y (2012) Quantitative Analysis of the Role Played by Poly (vinylpyrrolidone) in Seed-Mediated Growth of Ag Nanocrystals. J Am Chem Soc 134:1793–1801. https://doi.org/10.1021/ja210047e
Pu C, Zhao H, Hong Y, Zhan Q, Lan M (2020) Facile Preparation of Hydrophilic Mesoporous Metal-Organic Framework via Synergistic Etching and Surface Functionalization for Glycopeptides Analysis. Anal Chem 92:940–1947. https://doi.org/10.1021/acs.analchem.9b04236
Li K, Chen C, Chen C, Wang Y, Wei Z, Pan W, Song T (2015) Magnetosomes extracted from Magnetospirillum magneticum strain AMB-1 showed enhanced peroxidase-like activity under visible-light irradiation. Enzyme Microb Technol 72:72–78. https://doi.org/10.1016/j.enzmictec.2015.02.009
Yang J, Xiao X, Xia L, Li G, Shui L (2021) Microfluidic Magnetic Analyte Delivery Technique for Separation, Enrichment, and Fluorescence Detection of Ultratrace Biomarkers. Anal Chem 93:8273–8280. https://doi.org/10.1021/acs.analchem.1c01130
Zhou C, Cui K, Liu Y, Hao S, Zhang L, Ge S, Yu J (2021) Ultrasensitive Microfluidic Paper-Based Electrochemical/Visual Analytical Device via Signal Amplification of Pd@Hollow Zn/Co Core-Shell ZIF67/ZIF8 Nanoparticles for Prostate-Specific Antigen Detection. Anal Chem 93:5459–5467. https://doi.org/10.1021/acs.analchem.0c05134
Xia X, Zhang J, Lu N, Kim MJ, Ghale K, Xu Y, McKenzie E, Liu J, Ye H (2015) Quantitative Analysis of the Role Played by Poly(vinylpyrrolidone) in Seed-Mediated Growth of Ag Nanocrystals. ACS Nano 9:9994–10004. https://doi.org/10.1021/acsnano.5b03525
Ulf-Hakan S, Jari L, Zhang W, Patrik F (1999) Prostate-specific antigen. Semin Cancer Biol 9:83–93. https://doi.org/10.1006/scbi.1998.0086
Mark F, Stricker PD, Kaye KW (1997) Prostate cancer diagnosis and management. Lancet 349:1681–1687. https://doi.org/10.1016/S0140-6736(96)07393-X
Funding
Supported by the Fundamental Research Funds for the Central Universities (GK2021001007) and the Natural Science Foundation of Shaanxi Province (2018JM2005).
Author information
Authors and Affiliations
Contributions
Pengyue Sun and Yao Li are the first authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, P., Li, Y., Li, J. et al. Entrapment of horseradish peroxidase into nanometer-scale metal–organic frameworks: a new nanocarrier for signal amplification in enzyme-linked immunosorbent assay. Microchim Acta 188, 409 (2021). https://doi.org/10.1007/s00604-021-05065-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05065-9