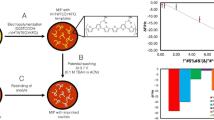
Abstract
An approach is reported based on the combination of aptamer and metal organic frameworks (MOF) to prepare a molecularly imprinted sensor that recognizes viruses with high specificity and sensitivity. Using MIL-101-NH2 as a polymer carrier, viral aptamers were introduced into the carrier surface through an amide reaction to specifically identify the target, and surface imprinting is carried out through tetraethyl silicate (TEOS) self-polymerization. The MIL-101-NH2 is also used as the reference fluorescence signal (λex/λem = 290/460 nm) and rhodamine B as the change signal (λex/λem = 550/570 nm). The ratiometric fluorescence detection and dual recognition strategy not only reduce environmental interference but also greatly improve the sensor’s anti-interference ability, the obtained imprinting factor was 5.72, and the detection limit as low as 1.8 pmol L−1. Therefore, the molecular imprinting sensor designed realizes the specific and highly sensitive identification of viruses, which provides theoretical support for the application of molecular imprinting technology in clinical diagnosis of viruses.

Aptamer-molecular imprinting polymer based on metal-organic framework ratiometric fluorescent detect virus.








Similar content being viewed by others
References
Chen GS, Kou XX, Huang SM, Huang SY, Zhang R, Liu C, Shen J, Zhu F, Ouyang GF (2018) llochroic-graphene oxide linked 3D oriented surface imprinting strategy for glycoproteins assays. Adv Funct Mater 28:1804129. https://doi.org/10.1002/adfm.201804129
Landry MP, Ando H, Chen AY, Cao J, Kottadiel VI, Chio L, Yang D, Dong J, Lu TK, Strano MS (2017) Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat Nanotechnol 12:368–377 https://www.nature.com/articles/nnano.2016.284
Li X, Scida K, Crooks RM (2015) Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal Chem 87:9009–9015. https://doi.org/10.1021/acs.analchem.5b02210
Huang J, Xie Z, Luo S, Xie L, Huang L, Fan Q, Zhang Y, Wang S, Zeng T (2016) Silver nanoparticles coated graphene electrochemical sensor for the ultrasensitive analysis of avian influenza virus H7. Anal Chim Acta 913:121–127. https://doi.org/10.1016/j.aca.2016.01.050
Dickert FL, Lieberzeit P, Tortschanoff M (2000) Molecular imprints as artificial antibodies — a new generation of chemical sensors. Sensor Actuator B-Chem 65:186–189. https://doi.org/10.1016/S0925-4005(99)00327-5
Hu R, Luan J, Kharasch ED, Singamaneni S, Morrissey JJ (2017) Aromatic functionality of target proteins influences monomer selection for creating artificial antibodies on plasmonic biosensors. ACS Appl Mater Interfaces 9:145–115. https://doi.org/10.1021/acsami.6b12505
Cumbo A, Lorber B, Corvini PFX, ssMeier W, Shahgaldian P (2013) A synthetic nanomaterial for virus recognition produced by surface imprinting. Nat Commun 4: 1925–1930. https://www.nature.com/articles/ncomms2529
Sykora S, Cumbo A, Belliot G (2015) Virus-like particles as virus substitutes to design artificial virus-recognition nanomaterials. Chem Commun 51:2256–2258. https://doi.org/10.1039/C4CC08843C
Li N, Liu Y, Liu F (2017) Bio-inspired virus imprinted polymer for prevention of viral infections. Acta Biomater 51:175–183. https://doi.org/10.1016/j.actbio.2017.01.017
Yang ZR, Wang MM, Wang XS, Yin XB (2017) Boric-acid-functional lanthanide metal−organic frameworks for selective ratiometric fluorescence detection of fluoride ions. Anal Chem 89:1930–1936. https://doi.org/10.1021/acs.analchem.6b04421
Lu Y, Yan B (2014) A ratiometric fluorescent pH sensor based on nanoscale metal–organic frameworks (MOFs) modified by europium (III) complex. Chem Commun 50:13323–13326. https://doi.org/10.1039/C4CC05508J
Micheroni D, Lan G, Lin W (2018) Efficient electrocatalytic proton reduction with carbon nano-tube-supported metal-organic frameworks. J Am Chem Soc 140:15591–15595. https://doi.org/10.1021/jacs.8b09521
Yin HQ, Yang J, Yin XB (2017) Ratiometric fluorescence sensing and real-time detection of water in organic solvents with one-pot synthesis of Ru@MIL-101(Al)-NH2. Anal Chem 89:13434–13440. https://doi.org/10.1021/acs.analchem.7b03723
Shinde S, El-Schich Z, Malakpour A, Wan W, Dizey N, Mo-hammadi R, Rurack K, Wingren AG, Sellergren B (2015) Sialic acid-imprinted fluorescent core–shell particles for selective labeling of cell surface glycans. J Am Chem Soc 137:13908–13912. https://doi.org/10.1021/jacs.5b08482
Li SH, Yin GH, Zhang Q, Li CL, Luo JH, Xu Z, Qin AL (2015) Selective detection of fenaminosulf via a molecularly imprinted fluorescence switch and silver nano-film amplification. Biosens Bioelectron 71:342–347. https://doi.org/10.1016/j.bios.2015.04.066
Mehrzad-Samarin M, Faridbod F, Dezfuli AS, Ganjali MR (2017) A novel metronidazole fluorescent nanosensor based on graphene quantum dots embedded silica molecularly imprinted polymer. Biosens Bioelectron 92:618–623. https://doi.org/10.1016/j.bios.2016.10.047
He K, Chen CC, Liang CS, Liu C, Yang B, Chen XM, Cai CQ (2016) Highly selective recognition and fluorescent detection of JEV via virus-imprinted magnetic silicon microspheres. Sens Actuators B-Chem 233:607–614. https://doi.org/10.1016/j.snb.2016.04.127
Yang B, Gong H, Chen CC, Chen XM, Cai CQ (2017) A virus resonance light scattering sensor based on mussel-inspired molecularly imprinted polymers for high sensitive and high selective detection of hepatitis a virus. Biosens Bioelectron 87:679–685. https://doi.org/10.1016/j.bios.2016.08.087
Xie J, Zhong G, Cai C, Chen C, Chen X (2017) Rapid and efficient separation of glycoprotein using pH double-responsive imprinted magnetic microsphere. Talanta 169:98–103. https://doi.org/10.1016/j.talanta.2017.03.065
Luo LH, Zhang F, Chen CC, Cai CQ (2019) Visual simultaneous detection of hepatitis a and B viruses based on a multifunctional molecularly imprinted fluorescence sensor. Anal Chem 91:15748–15756. https://doi.org/10.1021/acs.analchem.9b04001
Zhang ZJ, Liu JW (2019) Molecular imprinting with functional DNA. Small 15:1805246. https://doi.org/10.1002/smll.201805246
Zhang Z, Liu J (2016) Molecularly imprinted polymers with DNA aptamer fragments as macromonomers. ACS Appl Mater Interfaces 8:6371–6378. https://doi.org/10.1021/acsami.6b00461
Shahdostfard F, Roushani M (2017) Impedimetric detection of trinitrotoluene by using a glassy carbon electrode modified with a gold nanoparticle@fullerene composite and an aptamer-imprinted polydopamine. Microchim Acta 184:3997–4006. https://doi.org/10.1021/acsami.6b00461
Bai W, Gariano NA, Spivak DA (2013) Macromolecular amplification of binding response in superaptamer hydrogels. J Am Chem Soc 135:6977–6984. https://doi.org/10.1021/ja400576p
Tan JA, Guo ML, Tan L, Geng YY, Huang SY, Tang YW, Su CC, Lin CC, Liang Y (2018) Highly efficient fluorescent QDs sensor for specific detection of protein through double recognition of hybrid aptamer-molecular imprinted polymers. Sens Actuators B-Chem 274:627–635. https://doi.org/10.1016/j.snb.2018.07.126
Li W, Zhang Q, Wang YY, Ma YY, Guo ZC, Liu Z (2019) Controllably prepared aptamer-molecularly imprinted polymer hybrid for high-specificity and high-affinity recognition of target proteins. Anal Chem 91:4831–4837. https://doi.org/10.1021/acs.analchem.9b00465
Li CL, Li HK, Ge HJJ, Jie GF (2019) Versatile fluorescence detection of microRNA based on novel DNA hydrogel-amplified signal probes coupled with DNA walker amplification. Chem Commun 55:3919–3922. https://doi.org/10.1039/C9CC00565J
Ji XT, Wang JN, Niu SY, Ding CF (2019) A size-controlled DNA-cross-linked hydrogel coated silica nanoparticles served as ratio fluorescent probe for the detection of adenosine triphosphate in living cells. Chem Commun 55:5243–5246. https://doi.org/10.1039/C9CC01832H
Huang S, Wang LM, Zhu FW, Su W, Sheng JR, Huang CS, Xiao Q (2015) A ratiometric nanosensor based on fluorescent carbon dots for label-free and highly selective recognition of DNA. RSC Adv 5:44587–44597. https://doi.org/10.1039/C5RA05519A
Zu FL, Yan FY, Bai ZJ, Xu JX, Wang YY, Huang YC, Zhou XG (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Orte A, Alvarez-Pez JM, Ruedas-Rama MJ (2013) Ruedas-Rama fluorescence lifetime imaging microscopy for the detection of intracellular pH with quantum dot nanosensors. ACS Nano 7:6378–6395. https://doi.org/10.1021/nn402581q
Lu CH, Zhang Y, Tang SF, Yang ZBHG, Chen X, Chen GN (2012) Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens Bioelectron 31:439–444. https://doi.org/10.1016/j.bios.2011.11.008
Liang CS, Wang H, He K, Chen CY, Chen XM, Gong H, Cai CQ (2016) Virus-MIPs fluorescent sensor based on FRET for highly sensitive detection of JEV. Talanta 160:360–366. https://doi.org/10.1016/j.talanta.2016.06.010
Luo LH, Zhang F, Chen CY, Cai CQ (2020) Molecular imprinting resonance light scattering nanoprobes based on pH-responsive metal-organic framework for determination of hepatitis a virus. Microchim Acta 187:140–147. https://doi.org/10.1007/s00604-020-4122-1
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21775132), the National Natural Science Foundation of Hunan province (No. 2018JJ2388), Hunan 2011 Collaborative Innovation Center of Chemical Engineering & Technology with Environmental Benignity and Effective Resource Utilization, the project of the innovation team of the ministry of education (IRT_17R90).
Author information
Authors and Affiliations
Contributions
LW: methodology, conceptualization, writing—review and editing. JY: conceptualization, writing—review and editing. LT: methodology, validation, software, and editing. LL: methodology, conceptualization, software. CC: validation, data curation. HG: formal analysis, writing—review and editing. CC: writing–review and editing, supervision, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The Supporting Information is available free of charge on the ACS Publications website. Additional details of structure of XRD (Fig. S1), TGA (Fig. S2), N2 adsorption-desorption isotherm of (A) MIL-101-NH2 and (B) MIPs (Fig. S3). The influence of the amount of aptamer on IF and (F590/F460)0/(F590/F460) of MIPs and The influence of imprinting thickness on IF and (F590/F460)0/(F590/F460) of MIPs (Fig. S4). Optimization of the assay conditions (Fig. S5 - Fig. S9). Linearity of H-MIPs (Fig. S10), Fluorescence lifetime and UV–vis absorption spectrum of different species about MIP (Fig. S11), IF and SF (Table S1).
ESM 1
(DOCX 2.6 mb)
Rights and permissions
About this article
Cite this article
Wang, L., Yang, J., Tang, L. et al. Specific determination of HBV using a viral aptamer molecular imprinting polymer sensor based on ratiometric metal organic framework. Microchim Acta 188, 221 (2021). https://doi.org/10.1007/s00604-021-04858-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04858-2