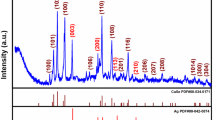
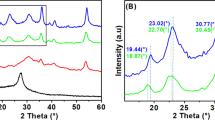
Abstract
A new electrochemical sensor is reported for the based on the application of noble bimetal nanoparticles (gold and copper) to polymeric-carbon-modifiers for the reduction of nitrate. This sensor was designed for nitrate ion measurement at the surface of pencil graphite electrode modified by a nanocomposite. The modification was the electrosynthesis of gold nanoparticles on the MWCNT/copper-polyaniline (Cu-PANI) nanocomposite. Physicochemical properties of the synthesized hybrid nanocomposites and their surface performance efficiency are characterized using microscopic, spectroscopic, and electrochemical techniques. At optimized pH, the nitrate peak current (at working potential of 1084 mV versus Ag/AgCl reference electrode) was linear in the concentration range 0.8–30.0 μM with a detection limit of 0.09 μM using differential pulse voltammetry. Modified sensor was successfully implemented to quantify nitrate ions in wastewater resulting from the production line for industrial barium chromate and an example of aqueduct water with appropriate recovery levels.
Graphical abstract

• Aniline was polymerized in phosphoric acid solution using peroxydisulfate as an initiator.
• MWCNT@CuNPs@PANNSs@AuNPs nanocomposite on PGE electrode was revealed specific recognition for NO3−.
• The electrochemical sensor displayed high selectivity and sensitivity for the detection of NO3−.







Similar content being viewed by others
References
Tang H, Sundari R, Lintang HO, Yuliati L (2016) Detection of nitrite and nitrate ions in water by graphene oxide as a potential fluorescence sensor. In: IOP Conference Series: Materials Science and Engineering, vol 1. IOP Publishing, Bristol, p 012027
Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N (2005) The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem 99(1):237–246
Liang J, Zheng Y, Liu Z (2016) Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water. Sensors Actuators B Chem 232:336–344
Van Zijderveld S, Gerrits W, Apajalahti J, Newbold J, Dijkstra J, Leng R, Perdok H (2010) Nitrate and sulfate: effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J Dairy Sci 93(12):5856–5866
Choi BC (1985) N-nfrroso compounds and human cancer: a molecular epidemiologic approach. Am J Epidemiol 121(5):737–743
Daniel WL, Han MS, Lee J-S, Mirkin CA (2009) Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J Am Chem Soc 131(18):6362–6363
Brender JD, Olive JM, Felkner M, Suarez L, Marckwardt W, Hendricks KA (2004) Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology 15:330–336
Badea M, Amine A, Palleschi G, Moscone D, Volpe G, Curulli A (2001) New electrochemical sensors for detection of nitrites and nitrates. J Electroanal Chem 509(1):66–72
WHO G (2011) Guidelines for drinking-water quality. World Health Organization 216:303–304
Li Y, Sun J, Bian C, Tong J, Xia S (2012) Micro electrochemical sensor with copper nanoclusters for nitrate determination in freshwaters. Micro Nano Lett 7(12):1197–1201
Motaghedifard MH, Pourmortazavi SM, Mirsadeghi S (2021) Selective and sensitive detection of Cr(VI) pollution in waste water via polyaniline/sulfated zirconium dioxide/multi walled carbon nanotubes nanocomposite based electrochemical sensor. Sensors Actuators B Chem 327:128882
Reyter D, Bélanger D, Roué L (2008) Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim Acta 53(20):5977–5984
Paixao TR, Cardoso JL, Bertotti M (2007) Determination of nitrate in mineral water and sausage samples by using a renewable in situ copper modified electrode. Talanta 71(1):186–191
Casella IG, Gatta M (2004) Electrochemical reduction of NO3− and NO2− on a composite copper thallium electrode in alkaline solutions. J Electroanal Chem 568:183–188
Artigas J, Jimenez C, Lemos S, Nogueira A, Torre-Neto A, Alonso J (2003) Development of a screen-printed thick-film nitrate sensor based on a graphite-epoxy composite for agricultural applications. Sensors Actuators B Chem 88(3):337–344
Zhang X, Wang J, Wang Z, Wang S (2005) Electrocatalytic reduction of nitrate at polypyrrole modified electrode. Synth Met 155(1):95–99
Koudehi MF, Pourmortazavi SM (2018) Polyvinyl alcohol/polypyrrole/molecularly imprinted polymer nanocomposite as highly selective chemiresistor sensor for 2,4-DNT vapor recognition. Electroanalysis 30(10):2302–2310
Wang Y, Qu J, Wu R, Lei P (2006) The electrocatalytic reduction of nitrate in water on Pd/Sn-modified activated carbon fiber electrode. Water Res 40(6):1224–1232
Tada K, Kawaguchi T, Shimazu K (2004) High electrocatalytic performance of Pd/Sn/Au electrodes for nitrate reduction. J Electroanal Chem 572(1):93–99
El-Deab MS (2004) Electrochemical reduction of nitrate to ammonia at modified gold electrodes. Electrochim Acta 49(9–10):1639–1645
Szpyrkowicz L, Daniele S, Radaelli M, Specchia S (2006) Removal of NO3− from water by electrochemical reduction in different reactor configurations. Appl Catal B Environ 66(1–2):40–50
da Rocha JRC, Angnes L, Bertotti M, Araki K, Toma HE (2002) Amperometric detection of nitrite and nitrate at tetraruthenated porphyrin-modified electrodes in a continuous-flow assembly. Anal Chim Acta 452(1):23–28
Shimazu K, Goto R, Piao S, Kayama R, Nakata K, Yoshinaga Y (2007) Reduction of nitrate ions on tin-modified palladium thin film electrodes. J Electroanal Chem 601(1–2):161–168
Brylev O, Sarrazin M, Roué L, Bélanger D (2007) Nitrate and nitrite electrocatalytic reduction on Rh-modified pyrolytic graphite electrodes. Electrochim Acta 52(21):6237–6247
De Groot M, Koper M (2004) The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J Electroanal Chem 562(1):81–94
Alam M, Hasnat M, Rashed M, Uddin SN, Rahman MM, Amertharaj S, Ahmed N, Mohamed N (2015) Nitrate detection activity of Cu particles deposited on pencil graphite by fast scan cyclic voltammetry. J Anal Chem 70(1):60–66
Fajerwerg K, Ynam V, Chaudret B, Garçon V, Thouron D, Comtat M (2010) An original nitrate sensor based on silver nanoparticles electrodeposited on a gold electrode. Electrochem Commun 12(10):1439–1441
Estudillo-Wong L, Arce-Estrada EM, Alonso-Vante N, Manzo-Robledo A (2011) Electro-reduction of nitrate species on Pt-based nanoparticles: surface area effects. Catal Today 166(1):201–204
Matsushima J, Silva W, Azevedo A, Baldan M, Ferreira N (2009) The influence of boron content on electroanalytical detection of nitrate using BDD electrodes. Appl Surf Sci 256(3):757–762
Sha R, Komori K, Badhulika S (2017) Graphene–polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim Acta 233:44–51
Rahimi-Nasrabadi M, Pourmortazavi SM, Ganjali MR, Reza Banan A, Ahmadi F (2014) Synthesis procedure optimization and characterization of europium (III) tungstate nanoparticles. J Mol Struct 1074:85–91
Liu B, Zou BX (2014) Electrocatalytic sensing of nitrate at Cu nanosheets electrodeposited on WO3/polyaniline modified electrode. In: Advanced Materials Research, vol. 881. Trans Tech Publications Ltd., Freienbach, pp 159–164
Pletcher D, Poorabedi Z (1979) The reduction of nitrate at a copper cathode in aqueous acid. Electrochim Acta 24(12):1253–1256
Dima G, Beltramo G, Koper M (2005) Nitrate reduction on single-crystal platinum electrodes. Electrochim Acta 50(21):4318–4326
Cattarin S (1992) Electrochemical reduction of nitrogen oxyanions in 1 M sodium hydroxide solutions at silver, copper and CuInSe 2 electrodes. J Appl Electrochem 22(11):1077–1081
Yang J, Chen J, Zhou Y, Wu K (2011) A nano-copper electrochemical sensor for sensitive detection of chemical oxygen demand. Sensors Actuators B Chem 153(1):78–82
Stortini A, Moretto L, Mardegan A, Ongaro M, Ugo P (2015) Arrays of copper nanowire electrodes: preparation, characterization and application as nitrate sensor. Sensors Actuators B Chem 207:186–192
Mirsadeghi S, Zandavar H, Yousefi M, Rajabi HR, Pourmortazavi SM (2020) Green-photodegradation of model pharmaceutical contaminations over biogenic Fe3O4/Au nanocomposite and antimicrobial activity. J Environ Manag 270:110831
Mirsadeghi S, Zandavar H, Rahimi M, Tooski HF, Rajabi HR, Rahimi-Nasrabadi M, Sohouli E, Larijani B, Pourmortazavi SM (2020) Photocatalytic reduction of imatinib mesylate and imipenem on electrochemical synthesized Al2W3O12 nanoparticle: optimization, investigation of electrocatalytic and antimicrobial activity. Colloids Surf A Physicochem Eng Asp 586:124254
Ullah R, Bilal S, Ali K (2014) Synthesis and characterization of polyaniline doped with Cu II chloride by inverse emulsion polymerization. Synth Met 198:113–117
Sahebi H, Pourmortazavi SM, Zandavar H, Mirsadeghi S (2019) Chitosan grafted onto Fe3O4@poly(N-vinylcaprolactam) as a new sorbent for detecting Imatinib mesylate in biosamples using UPLC-MS/MS. Analyst 144(24):7336–7350. https://doi.org/10.1039/C9AN01654F
Yan J, Wei T, Shao B, Fan Z, Qian W, Zhang M, Wei F (2010) Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 48(2):487–493
Pourmortazavi SM, Rahimi-Nasrabadi M, Karimi MS, Mirsadeghi S (2018) Evaluation of photocatalytic and supercapacitor potential of nickel tungstate nanoparticles synthesized by electrochemical method. New J Chem 42(24):19934–19944
Pourmortazavi SM, Taghdiri M, Makari V, Rahimi-Nasrabadi M (2015) Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim Acta A Mol Biomol Spectrosc 136:1249–1254
Huang H, Zhao M, Xing X, Bae I, Scherson D (1990) In-situ infrared studies of the Cd-UPD mediated reduction of nitrate on gold. J Electroanal Chem Interfacial Electrochem 293(1–2):279–284
Da Cunha M, Weber M, Nart FC (1996) On the adsorption and reduction of NO3− ions at Au and Pt electrodes studied by in situ FTIR spectroscopy. J Electroanal Chem 414(2):163–170
De D, Kalu EE, Tarjan PP, Englehardt JD (2004) Kinetic studies of the electrochemical treatment of nitrate and nitrite ions on iridium-modified carbon fiber electrodes. Chem Eng Technol 27(1):56–64. https://doi.org/10.1002/ceat.200401832
Khomutov N, Stamkulov U (1971) Nitrate reduction at various metal electrodes. Sov Electrochem 7:312–316
Bard AJ, Faulkner LR (2001) Fundamentals and applications. Electrochemical Methods 2(482):580–632
Hafezi B, Majidi MR (2013) A sensitive and fast electrochemical sensor based on copper nanostructures for nitrate determination in foodstuffs and mineral waters. Anal Methods 5(14):3552–3556
Mahmoudian M, Alias Y, Basirun W, MengWoi P, Jamali-Sheini F, Sookhakian M, Silakhori M (2015) A sensitive electrochemical nitrate sensor based on polypyrrole coated palladium nanoclusters. J Electroanal Chem 751:30–36
Gumpu MB, Nesakumar N, Ramachandra BL, Rayappan JBB (2017) Zinc oxide nanoparticles-based electrochemical sensor for the detection of nitrate ions in water with a low detection limit—a chemometric approach. J Anal Chem 72(3):316–326
Honarmand E, Motaghedifard MH, Hadi M, Mostaanzadeh H (2016) Electro-oxidation study of promethazine hydrochloride at the surface of modified gold electrode using molecular self assembly of a novel bis-thio Schiff base from ethanol media. J Mol Liq 216:429–439
Jonoush ZA, Rezaee A, Ghaffarinejad A (2020) Electrocatalytic nitrate reduction using Fe0/Fe3O4 nanoparticles immobilized on nickel foam: selectivity and energy consumption studies. J Clean Prod 242:118569
Choi P, Bessarabov DG, Datta R (2004) A simple model for solid polymer electrolyte (SPE) water electrolysis. Solid State Ionics 175(1–4):535–539
Daub K, Emig G, Chollier M-J, Callant M, Dittmeyer R (1999) Studies on the use of catalytic membranes for reduction of nitrate in drinking water. Chem Eng Sci 54(10):1577–1582
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motaghedifard, M.H., Pourmortazavi, S.M., Alibolandi, M. et al. Au-modified organic/inorganic MWCNT/Cu/PANI hybrid nanocomposite electrode for electrochemical determination of nitrate ions. Microchim Acta 188, 99 (2021). https://doi.org/10.1007/s00604-021-04754-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04754-9