Abstract
An electrochemical immunosensor for the determination of porcine epidemic diarrhea virus (PEDV) is described. It was manufactured by using gold nanoparticles/molybdenum disulfide/reduced graphene oxide nanocomposites modified on the surface of a glassy carbon electrode (GCE). The independently developed monoclonal antibody of PEDV-2C11 was immobilized on the modified electrode at site of gold nanoparticles provided in the nanocomposites. The concentration of PEDV was quantified by measuring the changes in the charge transfer resistance of the electrode before and after the immunoreaction between antigen-antibody by using hexacyanoferrate(II)/(III) as the redox probe. The frequency range was 10−1 to 105 Hz at the amplitude of 10 mV and an applied potential of + 0.180 V. Based on the immunoreaction between PEDV antigen and PEDV-2C11 antibody in 0.1 M phosphate buffer containing 0.1 M KCl at 37.5 °C for 140 min, the relative change in impedance was proportional to the logarithmic value of PEDV concentrations in the range of 82.5 to 1.65 × 104 TCID50 mL−1. Good reproducibility, stability, and specificity of the proposed immunosensor were obtained. It was successfully applied to the determination of PEDV in the spiked sample.

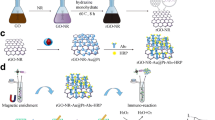
Schematic representation. a The preparation of AuNP/MoS2/rGO composites. b Representation of modification and functioning of the label-free electrochemical immunosensor and the electrochemical impedimetric response obtained before (a) and after (b) incubation of PEDV




Similar content being viewed by others
References
Pensaert MB, Bouck PD (1978) A new coronavirus-like particle associated with diarrhea in swine. Arch Virol 58:243–247. https://doi.org/10.1007/BF01317606
Ge FF, Yang DQ, Ju HB, Wang J, Liu J, Liu PH, Zhou JP (2013) Epidemiological survey of porcine epidemic diarrhea virus in swine farms in Shanghai, China. Arch Virol 158:2227–2231. https://doi.org/10.1007/s00705-013-1722-7
Mesquita JR, Honing HDR, Almeida A, Lourenco M, Poel WHM, Nascimento MSJ (2015) Outbreak of porcine epidemic diarrhea virus in Portugal. Transbound Emerg Dis 62:586–588. https://doi.org/10.1111/tbed.12409
Hofmann M, Wyler R (1989) Quantitation, biological and physicochemical properties of cell culture-adapted porcine epidemic diarrhea coronavirus (PEDV). Vet Microbiol 20:131–142. https://doi.org/10.1016/0378-1135(89)90036-9
Song D, Kang B, Lee S, Yang J, Moon H, Oh J, Ha G, Jang Y, Park B (2016) Use of an internal control in a quantitative RT-PCR assay for quantitation of porcine epidemic diarrhea virus shedding in pigs. J Virol Methods 133:27–33. https://doi.org/10.1016/j.jviromet.2005.10.021
Zhao PD, Bai J, Jiang P, Tang T, Li YF, Tan C, Shi XL (2014) Development of a multiplex TaqMan probe-based real-time PCR for discrimination of variant and classical porcine epidemic diarrhea virus. J Virol Methods 206:150–155. https://doi.org/10.1016/j.jviromet.2014.06.006
Okjin K, Chanhee C (2002) Comparison of reverse transcription polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine epidemic diarrhea virus in pigs. Can J Vet Res 66:112–116. https://doi.org/10.1080/03079450120118685
Bian HF, Xu F, Jia YM, Wang L, Deng SC, Jia AQ, Tang Y (2019) A new immunochromatographic assay for on-site detection of porcine epidemic diarrhea virus based on monoclonal antibodies prepared by using cell surface fluorescence immunosorbent assay. BMC Vet Res 15(32):1–10. https://doi.org/10.1186/s12917-019-1773-4
Labib M, Shipman PO, Martic S, Kraatz H (2011) Towards an early diagnosis of HIV infection: an electrochemical approach for detection of HIV-1 reverse transcriptase enzyme. Analyst 136:708–715. https://doi.org/10.1039/c0an00741b
Carneiro P, Loureiro J, Delerue-Matos C, Morais S, Pereira MDC (2017) Alzheimer’s disease: development of a sensitive label-free electrochemical immunosensor for detection of amyloid beta peptide. Sensors Actuators B 239:157–165. https://doi.org/10.1016/j.snb.2016.07.181
Eissa S, Chinnappan R, Zourob M (2017) Ultrasensitive label-free electrochemical immunosensors for multiple cell surface biomarkers on liver cancer stem cells. Electroanalysis 29:1994–2000. https://doi.org/10.1002/elan.201700016
Yu LL, Zhang Y, Hu CY, Wu H, Yang YY, Huang CS, Jia NQ (2015) Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem 176:22–26. https://doi.org/10.1016/j.foodchem.2014.12.030
Yang GJ, Jin WJ, Wu LP, Wang QQ, Shao HX, Qin AJ, Yu B, Li DM, Cai BL (2011) Development of an impedimetric immunosensor for the determination of 3-amino-2-oxazolidone residue in food samples. Anal Chim Acta 706:120–127. https://doi.org/10.1016/j.aca.2011.08.018
Dai YP, Wang T, Hu XY, Liu SZ, Zhang M, Wang CY (2017) Highly sensitive microcantilever-based immunosensor for the detection of carbofuran in soil and vegetable samples. Food Chem 229:432–438. https://doi.org/10.1016/j.foodchem.2017.02.093
Afkhami A, Hashemi P, Bagheri H, Salimian J, Ali A, Madrakian T, Hashemi P, Bagheri H (2017) Impedimetric immunosensor for the label-free and direct detection of botulinum neurotoxin serotype A using Au nanoparticles/graphene-chitosan composite. Biosens Bioelectron 93:124–131. https://doi.org/10.1016/j.bios.2016.09.059
Lu ZW, Zhang JJ, Dai WL, Lin XN, Ye JP, Ye JS (2017) A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Microchim Acta 183:4731–4740. https://doi.org/10.1007/s00604-017-2521-8
Hasanpour F, Taei M, Tahmasebi S (2018) Ultra-sensitive electrochemical sensing of acetaminophen and codeine in biological fluids using CuO/CuFe2O4 nanoparticles as a novel electrocatalyst. J Food Drug Anal 26:879–886. https://doi.org/10.1016/j.jfda.2017.10.001
Belkhamssa N, Justino CIL, Santos PSM, Cardoso S, Lopes I, Duarte AC, Rocha-Santos T, Ksibi M (2016) Label-free disposable immunosensor for detection of atrazine. Talanta 146:430–434. https://doi.org/10.1016/j.talanta.2015.09.015
Lei YG, Yang C, Hou JH, Wang F, Min SX, Ma XH, Jin ZL, Xu J, Lu GX, Huang KW (2017) Strongly coupled CdS/graphene quantum dots nanohybrids for highly efficient photocatalytic hydrogen evolution: unraveling the essential roles of graphene quantum dots. Appl Catal B Environ 216:59–69. https://doi.org/10.1016/j.apcatb.2017.05.063
Chen HF, Tang DP, Zhang B, Liu B-Q, Cui YL, Chen GN (2012) Electrochemical immunosensor for carcinoembryonic antigen based on nanosilver-coated magnetic beads and gold-graphene nanolabels. Talanta 91:95–102. https://doi.org/10.1016/j.talanta.2012.01.025
Kourosh KZ, Jian ZN (2016) Biosensors based on two-dimensional MoS2. ACS Sensor 1:5–16. https://doi.org/10.1021/acssensors.5b00142
Wang YY, Chen F, Ye XX, Wu T, Wu KB, Li CY (2017) Photoelectrochemical immunosensing of tetrabromobisphenol based on the enhanced effect of dodecahedral Au nanocrystals/MoS2 nanosheets. Sensors Actuators B 245:205–212. https://doi.org/10.1016/j.snb.2017.01.140
Vilian AE, Dinesh B, Kang SM , Krishnan UM , Huh YS, Han YK(2019) Recent advances in molybdenum disulfide-based electrode materials for electroanalytical applications. Microchim Acta 186:203. https://doi.org/10.1007/s00604-019-3287-y
Pumera M, Loo AH (2014) Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. Trends Anal Chem 61:49–53. https://doi.org/10.1016/j.trac.2014.05.009
Loo AH, Bonanni A, Ambrosi A, Pumera M (2014) Molybdenum disulfide (MoS2) nanoflakes as inherently electroactive labels for DNA hybridization detection. Nanoscale 6:11971–11975. https://doi.org/10.1039/c4nr03795b
Wang XX, Nan FX, Zhao JL, Yang T, Ge T, Kui J (2015) A label-free ultrasensitive electrochemical DNA sensor based on thin-layer MoS2 nanosheets with high electrochemical activity. Biosens Bioelectron 64:386–391. https://doi.org/10.1016/j.bios.2014.09.030
Zhang G, Liu HJ, Qu JH, Li J (2016) Two-dimensional layered MoS2: rational design, properties and electrochemical applications. Energ Environ Sci 9:1190–1209. https://doi.org/10.1039/c5ee03761a
Kim JY, Byun S, Smith AJ, Yu J, Huang JX (2013) Enhanced electrocatalytic properties of transition-metal dichalcogenides sheets by spontaneous gold nanoparticle decoration. J Phys Chem Lett 4:1227–1232. https://doi.org/10.1021/jz400507t
Wang Q, Tu YD, Ichii T, Utsunomiya T, Sugimura H, Hao LF, Wang RG, He XD (2017) Decoration of reduced graphene oxide by Au nanoparticles: an enhanced negative photoconductivity. Nanoscale 38:14703–14709. https://doi.org/10.1039/c7nr05143c
Su S, Cao WF, Liu W, Lu ZW, Zhu D, Chao J, Weng LX, Wang LH, Fan CH, Wang L-H (2017) Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens Bioelectron 94:552–559. https://doi.org/10.1016/j.bios.2017.03.040
Wang YG, Wang YL, Wu D, Ma HM, Zhang Y, Fan DW, Pang XH, Du B, Wei Q (2018) Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sensors Actuators B 255:125–132. https://doi.org/10.1016/j.snb.2017.07.129
Frence G, Kolloid Z (1973) Controlled nucleation for the regulation of the particle size in monodisperse Au suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Chekina F, Bagga K, Subramanian P, Jijie R, Singhe SK, Kurungote S, Boukherroub R, Szunerits S (2018) Nucleic aptamer modified porous reduced graphene oxide/MoS2 based electrodes for viral detection: application to human papillomavirus (HPV). Sensors Actuators B 262:991–1000. https://doi.org/10.1016/j.snb.2018.02.065
Zhang YL, Chen M, Li HY, Yan FQ, Pang PF, Wang HB, Wu Z, Yang WR (2017) A molybdenum disulfide/gold nanorod composite-based electrochemical immunosensor for sensitive and quantitative detection of microcystin-LR in environmental samples. Sensors Actuators B 244:606–615. https://doi.org/10.1016/j.snb.2017.01.030
Alarfaj NA, El-Tohamy MF, Orabyc H (2018) New label-free ultrasensitive electrochemical immunosensor-based Au/MoS2/rGO nanocomposites for CA 27-29 breast cancer antigen detection. New J Chem 42:11046–11053. https://doi.org/10.1039/c8nj01388h
Sun YQ, Ma ZY, Yan RQ, Wang DF, Cao WW, Guo YP (2016) Establishment and application of duplex RT-PCR assay for detection of porcine epidemic diarrhea virus and pseudorabies virus. China Anim Husb Vet Med 43:2455–2460. https://doi.org/10.16431/j.cnki.1671-7236.2016.09.034
Wang YY, Zhao ZP, Zhao W, Wang J, Zheng JG, Jiang JH, Chai WX, Qin AJ, Jin WJ (2018) Establishment of a colloidal gold detection method for porcine epidemic diarrhea virus. Anim Husb Vet Med 50:100–105
Acknowledgments
The authors are thankful to Prof. Ming Shen, School of Chemistry and Chemical Engineering, Yangzhou University, for assistance in material synthesis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 116 kb)
Rights and permissions
About this article
Cite this article
Li, X., Wang, Y., Zhang, X. et al. An impedimetric immunosensor for determination of porcine epidemic diarrhea virus based on the nanocomposite consisting of molybdenum disulfide/reduced graphene oxide decorated with gold nanoparticles. Microchim Acta 187, 217 (2020). https://doi.org/10.1007/s00604-020-4166-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4166-2