Abstract
A magnetic nanocomposite adsorbent based on Zn-Al layered double hydroxide (LDH) intercalated with tyrosine has been synthesized for ultrasound-assisted extraction of two drugs of abuse: tramadol (TRA) and methadone (MET). Analysis was carried out using gas chromatography-mass spectrometry. The synthesized LDH was characterized by Fourier transform-infrared spectroscopy, X-ray diffraction, field emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The most important extraction parameters such as type of the elution solvent, pH value of the sample solution, and the amount of the adsorbent were optimized. With assistance of ultrasound radiation, the maximum extraction of target drugs using the fabricated LDH was achieved within 5 min. Under the optimized conditions, the limits of determination were 0.45, 0.45, 2.5, and 0.8 μg L−1 for TRA and 0.15, 0.15, 1.2, and 0.5 μg L−1 for MET in water, urine, plasma, and saliva samples, respectively. The preconcentration factors obtained were in the range of 50–145. The matrix effect for MET and TRA is considerable in plasma (66%, 18%) and saliva (72%, 34%), respectively. The precision was found to be better 11% RSD. The maximum adsorption capacity is 4.84 (mg g−1) (L mg−1)1/n based on the Freundlich isotherm. The proposed method presents good results for trace determination of tramadol and methadone in biological samples with satisfactory repeatability.

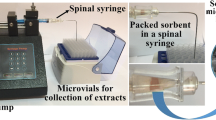
Graphical abstract




Similar content being viewed by others
References
Dole VP, Nyswander ME (1976) Methadone maintenance treatment: A Ten- Year perspective. J Amer Med Assoc 235:2117–2119 1001/jama.1976.03260450029025
Baselt RC (2009) Disposition of toxic drugs and chemicals in man, 8th edn. Biomedical Publications, Foster City, CA, pp 941–945
Centers for Disease Control and Prevention (2019) Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Accessed 2 May 2019
Moeller KE, Lee KC, Kissack JC (2008) Urine drug screening: practical guide for clinicians. Mayo Clin Proc 83:66–76. https://doi.org/10.4065/83.1.66
Brettell TA, Lum BJ (2018) Analysis of drugs of abuse by gas chromatography-mass spectrometry (GC-MS). Methods Mol Biol 1810:29–42. https://doi.org/10.1007/978-1-4939-8579-1-3
Tanaka H, Naito T, Mino Y, Kawakami J (2016) Validated determination method of tramadol and its desmethylates in human plasma using an isocratic LC-MS/MS and its clinical application to patients with cancer pain or non-cancer pain. J Pharm Health Care Sci 4:2–25. https://doi.org/10.1186/s40780-016-0059-2
Cheng PS, Lee CH, Tiu C, Chien CS (2008) Simultaneous determination of ketamine, tramadol, methadone, and their metabolites in urine by gas chromatography-mass spectrometry. J Anal Toxicol 32:253–259. https://doi.org/10.1093/jat/32.3.253
Sheibani A, Haghpazir N (2014) Application of ion mobility spectrometry for the determination of tramadol in biological samples. J Food Drug Anal 22:500–504. https://doi.org/10.1016/j.jfda.2014.02.001
Lucas ACS, Bermejo AM, Tabernero MJ, Fern’andez P, Strano-Rossi S (2000) Use of solid-phase microextraction (SPME) for the determination of methadone and EDDP in human hair by GC–MS. Forensic Sci Int 107:225–232. https://doi.org/10.1016/S0379-0738(99)00165-6
Rosado T, Barroso M, Vieira DN, Gallardo E (2019) Determination of selected opiates in hair samples using microextraction by packed sorbent: a new approach for sample clean-up. J Anal Toxicol 43:465–476. https://doi.org/10.1093/jat/bkz029
Gorynski K (2019) A critical review of solid-phase microextraction applied in drugs of abuse determinations and potential applications for targeted doping testing. TrAC-Trend Anal Chem 112:135–146. https://doi.org/10.1016/j.trac.2018.12.029
Nouri N, Khorram P, Sereshti H (2019) Applications of three-dimensional graphenes for preconcentration, extraction, and sorption of chemical species:a review. Microchim Acta 186:232. https://doi.org/10.1007/s00604-019-3324-x
Hasheminasab KS, Fakhari AR, Koruni MH (2014) Development of carbon-nanotube-assisted electromembrane extraction in the two-phase mode combined with GC for the determination of basic drugs. J Sep Sci 37:85–91. https://doi.org/10.1002/jssc.201300480
Britto S, Radha AV, Ravishankar N, Kamath PV (2007) Solution decomposition of the layered double hydroxide (LDH) of Zn with Al. Solid State Sci 9:279–286. https://doi.org/10.1016/j.solidstatesciences.2007.01.00
Fan G, Li F, Evans DG, Duan X (2014) Catalytic applications of layered double hydroxides: recent advances and perspectives. Chem Soc Rev 43:7040–7066. https://doi.org/10.1039/C4CS00160E
Shao M, Ning F, Zhao J, Wei M, Evans DG, Duan X (2012) Preparation of Fe3O4@SiO2@layered double hydroxide core−shell microspheres for magnetic separation of proteins. J Am Chem Soc 134:1071–1077. https://doi.org/10.1021/ja2086323
Asiabi H, Yamini Y, Shamsayei M (2018) Using cobalt/chromium layered double hydroxide nano-sheets as a novel packed in-tube solid phase microextraction sorbent for facile extraction of acidic pesticides from water samples. New J Chem 42: 9935–9944. https://doi.org/0.1039/ C8NJ00372F
Musumeci AW, Mortimer GM, Butler MK, Xu ZP, Minchin RF, Martin DJ (2010) Fluorescent layered double hydroxide nanoparticles for biological studies. Appl Clay Sci 48:271–279. https://doi.org/10.1016/j.clay.2009.11.008
Chubar N, Gilmour R, Gerda V, Mičušík M, Omastova M, Heister K, Man P, Fraissard J, Zaitsev V (2017) Layered double hydroxides as the next generation inorganic anion exchangers: synthetic methods versus applicability. Adv Colloid Interf Sci 245:62–80. https://doi.org/10.1016/j.cis.2017.04.013
Choy JH, Kwak SY, Park JS, Jeong YJ, Portier J (1999) Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J Am Chem Soc 121:1399–1400. https://doi.org/10.1021/ja981823f
Choy JH, Kwak SY, Jeong YJ, Park JS (2000) Inorganic layered double hydroxides as nonviral vectors. Angew Chem Int Ed 39:4041–4045. https://doi.org/10.1002/1521-3773(20001117)39:22<4041::AID-ANIE4041>3.0.CO;2-C
Nakayama H, Wada N, Tsuhako M (2004) Intercalation of amino acids and peptides into Mg–Al layered double hydroxide by reconstruction method. Int J Pharm 269:469–478. https://doi.org/10.1016/j.ijpharm.2003.09.043
Aisawa S, Kudo H, Hoshi T, Takahashi S, Hirahara H, Umetsu Y, Narita E (2004) Intercalation behavior of amino acids into Zn–Al layered double hydroxide by calcination–rehydration reaction. J Solid State Chem 177:3987–3994. https://doi.org/10.1016/j.jssc.2004.07.024
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications: a review. Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Adlnasab L, Ezoddin M, Karimi MA, Hatamikia N (2018) MCM-41@Cu–Fe–LDH magnetic nanoparticles modified with cationic surfactant for removal of Alizarin Yellow from water samples and its determination with HPLC. Res Chem Intermed 44:3249–3265. https://doi.org/10.1007/s11164-018-3304-5
Liu X, Ma Z, Xing J, Liu H (2004) Preparation and characterization of amino–silane modified superparamagnetic silica nanospheres. J Magn Magn Mater 270:1–6. https://doi.org/10.1016/j.jmmm.2003.07.006
Naderi Kalali E, Wang X, Wang DY (2016) Synthesis of Fe3O4 nano-sphere@MgAl layered double hydroxide hybrid and application in the fabrication of multifunctional epoxy nanocomposites. Ind Eng Chem Res 55:6634–6642. https://doi.org/10.1021/acs.iecr.5b04873
Adlnasab L, Ezoddin M, Shabanian M, Mahjoob B (2019) Development of ferrofluid mediated CLDH@Fe3O4@Tanic acid- based supramolecular solvent: application in air-assisted dispersive micro solid phase extraction for preconcentration of diazinon and metalaxyl from various fruit juice samples. Microchem J 146:1–11. https://doi.org/10.1016/j.microc.2018.12.020
Kloprogge JT, Wharton D, Hickey L (2002) Infrared and Raman study of interlayer anions CO32−, NO3−, SO42− and ClO4− in Mg/Al hydrotalcite. Am Mineral 87:623–629. https://doi.org/10.2138/am-2002-5-604
Yan L, Yang K, Shan R, Yu H, Du B (2015) Calcined ZnAl- and Fe3O4/ZnAl–layered double hydroxides for efficient removal of Cr (VI) from aqueous solution. RSC Adv 5:96495–96503. https://doi.org/10.1039/C5RA17058C
Bruni S, Cariati F, Casu M, Lai A, Musinu A, Piccaluga G, Solinas S (1999) IR and NMR study of nanoparticle–support interactions in a Fe2O3–SiO2 nanocomposite prepared by a sol–gel method. Nano Struct Mater 11:573–586. https://doi.org/10.1016/S0965-9773(99)00335-9
Yan L, Yang K, Shan R, Yan T, Wei J, Yu S, Yu H, Du B (2015) Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J Colloid Interface Sci 448:508–516. https://doi.org/10.1016/j.jcis.2015.02.048
De Roy A, Forano C, Besse JP (2001) In: Rives V (ed) Layered double hydroxides: present and future. Nova Science Publishers, New York, p 12
Tang S, Lee HK (2013) Application of dissolvable layered double hydroxides as sorbent in dispersive solid-phase extraction and extraction by co-precipitation for the determination of aromatic acid anions. Anal Chem 85:7426–7433. https://doi.org/10.1021/ac4013573
Caban M, Migowska N, Stepnowski P, Kwiatkowski M, Kumirska J (2012) Matrix effects and recovery calculations in analyses of pharmaceuticals based on the determination of β-blockers and -agonists in environmental samples. J Chromatogr A 1258 (2012) 117–127. https://doi.org/10.1021/ac971078+
Fakhari AR, Asadi S, Mohammadi Kosalar H, Sahragard A, Hashemzadeh A, Amini MM (2017) Metal-organic framework enhanced electromembrane extraction: a conceptual study using basic drugs as model substances. Anal Methods 9:5646–5652. https://doi.org/10.1039/C7AY01093A
Acknowledgments
This investigation by the Standard Research Institute (Karaj, Iran), Iran National Science Foundation (INSF, project no. 96012135), Borujerd branch of Islamic Azad University, and University of Zabol are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 700 kb)
Rights and permissions
About this article
Cite this article
Adlnasab, L., Shahdousti, P. & Ahmar, H. Layered double hydroxide intercalated with tyrosine for ultrasonic-assisted microextraction of tramadol and methadone from biological samples followed by GC/MS analysis. Microchim Acta 187, 265 (2020). https://doi.org/10.1007/s00604-020-04237-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04237-3