Abstract
Simultaneous fluorometric determination of iron(III) and copper(II) without the use of any masking agent or additional treatment is achieved by using N-doped carbon dots (NCDs). The NCDs were hydrothermally prepared, have strongest excitation/emission peaks at 320/406 nm and a 47% quantum yield. Excitation-tunable emission is found to depend on solution pH values. This supports the involvement of surface states in the origin of the excitation dependent nature. The NCDs were employed as a fluorescent probe for the simultaneous determination of Fe(III) with a linear response in the 3–60 μM concentration range and a 0.31 μM detection limit (LOD). The probe also responds linearly to Cu(II) in the 0.5–15 μM concentration range and with a 56 nM LOD. With the addition of Cu(II), the absorption spectra of NCDs presented a clear decrease in the intensity at 312 nm followed by an increase at 360 nm. This is not observed in the presence of Fe(III). The fluorescence lifetime of NCDs (5.8 ns) is reduced by Fe(III) but not by Cu(II). Thus, the two metal ions can be simultaneously detected without the need for any reagents. The probe was employed to quantify Fe(III) and Cu(II) in spiked water, serum, and urine samples.

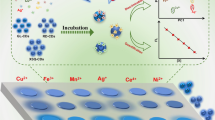
Schematic representation of hydrothermal synthesis of highly fluorescent N-doped carbon dots with novel pH dependent emission and their application for the simultaneous determination of Cu(II) and Fe(III) with individual ion discrimination.








Similar content being viewed by others
References
Tapiero H, Townsend DM, Tew KD (2003) Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 57:386–398. https://doi.org/10.1016/S0753-3322(03)00012-X
Aisen P, Wessling-Resnick M, Leibold EA (1999) Iron metabolism. Curr Opin Chem Biol 3:200–206. https://doi.org/10.1016/S1367-5931(99)80033-7
Zhou X, Zhao G, Tan X et al (2019) Nitrogen-doped carbon dots with high quantum yield for colorimetric and fluorometric detection of ferric ions and in a fluorescent ink. Microchim Acta 186:67. https://doi.org/10.1007/s00604-018-3176-9
Zhao L, Li H, Xu Y et al (2018) Selective detection of copper ion in complex real samples based on nitrogen-doped carbon quantum dots. Anal Bioanal Chem 410:4301–4309. https://doi.org/10.1007/s00216-018-1079-6
Bandi R, Dadigala R, Gangapuram BR, Guttena V (2018) Green synthesis of highly fluorescent nitrogen – doped carbon dots from Lantana camara berries for effective detection of lead(II) and bioimaging. J Photochem Photobiol B Biol 178:330–338. https://doi.org/10.1016/j.jphotobiol.2017.11.010
Kou S, Nam SW, Shumi W et al (2009) Microfluidic detection of multiple heavy metal ions using fluorescent Chemosensors. Bull Kor Chem Soc 30:1173–1176. https://doi.org/10.5012/bkcs.2009.30.5.1173
Sun X, Lei Y (2017) Fluorescent carbon dots and their sensing applications. TrAC - Trends Anal Chem 89:163–180. https://doi.org/10.1016/j.trac.2017.02.001
Gao X, Du C, Zhuang Z, Chen W (2016) Carbon quantum dot-based nanoprobes for metal ion detection. J Mater Chem C 4:6927–6945. https://doi.org/10.1039/c6tc02055k
Gao W, Song H, Wang X et al (2018) Carbon Dots with Red Emission for Sensing of Pt 2+ , Au 3+ , and Pd 2+ and Their Bioapplications in Vitro and in Vivo. ACS Appl Mater Interfaces 10:1147–1154. https://doi.org/10.1021/acsami.7b16991
Zhu X, Wang J, Zhu Y et al (2018) Green emitting N,S-co-doped carbon dots for sensitive fluorometric determination of Fe(III) and Ag(I) ions, and as a solvatochromic probe. Microchim Acta 185:510. https://doi.org/10.1007/s00604-018-3045-6
Ren G, Zhang Q, Li S et al (2017) One pot synthesis of highly fluorescent N doped C-dots and used as fluorescent probe detection for Hg2+ and Ag+ in aqueous solution. Sensors Actuators B Chem 243:244–253. https://doi.org/10.1016/j.snb.2016.11.149
Singh AK, Singh VK, Singh M et al (2019) One pot hydrothermal synthesis of fluorescent NP-carbon dots derived from Dunaliella salina biomass and its application in on-off sensing of Hg (II), Cr (VI) and live cell imaging. J Photochem Photobiol A Chem 376:63–72. https://doi.org/10.1016/j.jphotochem.2019.02.023
Zhang S, Li J, Zeng M et al (2014) Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 6:4157–4162. https://doi.org/10.1039/c3nr06744k
Wang B, Tan H, Zhang T et al (2019) Hydrothermal synthesis of N-doped carbon dots from an ethanolamine-ionic liquid gel to construct label-free multifunctional fluorescent probes for Hg 2+ , Cu 2+ and S 2 O 32. Analyst 144:3013–3022. https://doi.org/10.1039/c9an00116f
Patir K, Gogoi SK (2019) Nitrogen-doped carbon dots as fluorescence ON–OFF–ON sensor for parallel detection of copper( ii ) and mercury( ii ) ions in solutions as well as in filter paper-based microfluidic device. Nanoscale Adv 1:592–601. https://doi.org/10.1039/c8na00080h
Yu J, Song N, Zhang Y-K et al (2015) Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sensors Actuators B Chem 214:29–35. https://doi.org/10.1016/j.snb.2015.03.006
Ye Q, Yan F, Luo Y et al (2017) Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion. Spectrochim Acta - Part A Mol Biomol Spectrosc 173:854–862. https://doi.org/10.1016/j.saa.2016.10.039
Li C, Liu W, Ren Y et al (2017) The selectivity of the carboxylate groups terminated carbon dots switched by buffer solutions for the detection of multi-metal ions. Sensors Actuators B Chem 240:941–948. https://doi.org/10.1016/j.snb.2016.09.068
Luo L, Wang P, Wang Y, Wang F (2018) pH assisted selective detection of Hg(II) and Ag(I) based on nitrogen-rich carbon dots. Sensors Actuators B Chem 273:1640–1647. https://doi.org/10.1016/j.snb.2018.07.090
Sharma V, Saini AK, Mobin SM (2016) Multicolour fluorescent carbon nanoparticle probes for live cell imaging and dual palladium and mercury sensors. J Mater Chem B 4:2466–2476. https://doi.org/10.1039/C6TB00238B
Roy P, Chen PC, Periasamy AP et al (2015) Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today 18:447–458. https://doi.org/10.1016/j.mattod.2015.04.005
Ramanan V, Thiyagarajan SK, Raji K et al (2016) Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging. ACS Sustain Chem Eng 4:4724–4731. https://doi.org/10.1021/acssuschemeng.6b00935
Bandi R, Gangapuram BR, Dadigala R et al (2016) Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv 6:28633–28639. https://doi.org/10.1039/c6ra01669c
Ramanan V, Siddaiah B, Raji K, Ramamurthy P (2018) Green synthesis of multifunctionalized, nitrogen-doped, highly fluorescent carbon dots from waste expanded polystyrene and its application in the Fluorimetric detection of Au3+ ions in aqueous media. ACS Sustain Chem Eng 6:1627–1638. https://doi.org/10.1021/acssuschemeng.7b02852
Bandi R, Devulapalli NP, Dadigala R et al (2018) Facile Conversion of Toxic Cigarette Butts to N,S-Codoped Carbon Dots and Their Application in Fluorescent Film, Security Ink, Bioimaging, Sensing and Logic Gate Operation. ACS Omega 3:13454–13466. https://doi.org/10.1021/acsomega.8b01743
Sharma A, Gadly T, Gupta A et al (2016) Origin of excitation dependent fluorescence in carbon Nanodots. J Phys Chem Lett 7:3695–3702. https://doi.org/10.1021/acs.jpclett.6b01791
Sharma A, Gadly T, Neogy S et al (2017) Molecular origin and self-assembly of fluorescent carbon Nanodots in polar solvents. J Phys Chem Lett 8:1044–1052. https://doi.org/10.1021/acs.jpclett.7b00170
Wang W, Peng J, Li F et al (2019) Phosphorus and chlorine co-doped carbon dots with strong photoluminescence as a fluorescent probe for ferric ions. Microchim Acta 186:32–39. https://doi.org/10.1007/s00604-018-3140-8
Sun Y, Wang X, Wang C et al (2018) Red emitting and highly stable carbon dots with dual response to pH values and ferric ions. Microchim Acta 185:83. https://doi.org/10.1007/s00604-017-2544-1
Atchudan R, Edison TNJI, Aseer KR et al (2018) Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens Bioelectron 99:303–311. https://doi.org/10.1016/j.bios.2017.07.076
Deng M, Wang S, Liang C et al (2016) A FRET fluorescent nanosensor based on carbon dots for ratiometric detection of Fe 3+ in aqueous solution. RSC Adv 6:26936–26940. https://doi.org/10.1039/c6ra02679f
Rong MC, Zhang KX, Wang YR, Chen X (2017) The synthesis of B, N-carbon dots by a combustion method and the application of fluorescence detection for Cu 2+. Chin Chem Lett 28:1119–1124. https://doi.org/10.1016/j.cclet.2016.12.009
Wang ZX, Yu XH, Li F et al (2017) Preparation of boron-doped carbon dots for fluorometric determination of Pb(II), Cu(II) and pyrophosphate ions. Microchim Acta 184:4775–4783. https://doi.org/10.1007/s00604-017-2526-3
Chen J, Li Y, Lv K et al (2016) Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sensors Actuators B Chem 224:298–306. https://doi.org/10.1016/j.snb.2015.10.046
Wang Y, Wu WT, Wu MB et al (2015) Yellow-visual fluorescent carbon quantum dots from petroleum coke for the efficient detection of Cu2+ ions. Xinxing Tan Cailiao/New Carbon Mater 30:550–559. https://doi.org/10.1016/S1872-5805(15)60204-9
Acknowledgements
One of the authors (Rajkumar Bandi) gratefully acknowledges CSIR-INDIA for Senior Research Fellowship. The authors are grateful to MRC, MNIT Jaipur for extending various analytical facilities. The authors would like to thank DST-FIST, New Delhi, India for providing necessary analytical facilities in the department. Sincere thanks to Department of Chemistry, Osmania University (OU) and CFRD-OU for providing infrastructure and other necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical standards and informed consent
The urine sample was obtained from a healthy volunteer in Osmania University and informed consent was obtained for the use of human urine. Human serum sample was obtained from Osmania General Hospital (OGH) and appropriate permissions were taken from the relevant authorities of OGH for the use of human serum sample. This research was approved by Osmania University Ethical committee and all experiments were performed in accordance with the guideline for experimentation of Osmania University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4.77 MB)
Rights and permissions
About this article
Cite this article
Bandi, R., Dadigala, R., Gangapuram, B.R. et al. N-Doped carbon dots with pH-sensitive emission, and their application to simultaneous fluorometric determination of iron(III) and copper(II). Microchim Acta 187, 30 (2020). https://doi.org/10.1007/s00604-019-4017-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4017-1