Abstract
A photocathode is described for the determination of microRNA-21 by using CuInS2 as an active photocathode material. Exonuclease III assisted target recycling amplification was employed to enhance the detection sensitivity. The TATA-binding protein (TBP) was applied to enhance steric hindrance which decreases the photoelectrochemical intensity. This strategy is designed by combining the anti-interference photocathode material, enzyme assisted target recycling amplification and TBP induced signal off, showing remarkable amplification efficiency. Under the optimized conditions, the detection limit for microRNA-21 is as low as 0.47 fM, and a linear range was got from 1.0 × 10−15 M to 1.0 × 10−6 M.

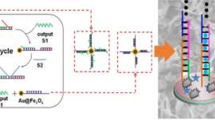
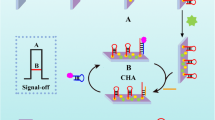
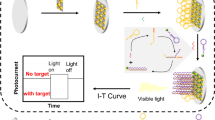
Schematic representation of sensitive photoelectrochemical detection of microRNA-21.CuInS2 is used as an active photocathode material. Combined Exonuclease III assisted target recycling amplification and TATA-binding protein decreased of photoelectrochemical intensity, the detection limit was 0.47 fM with good selectivity. (miR-21: microRNA-21; CS: chitosan).






Similar content being viewed by others
References
Zhao WW, Xu JJ, Chen HY (2015) Photoelectrochemical bioanalysis: the state of the art. Chem Soc Rev 46:729–741
Qu YQ, Duan XF (2013) Progress, challenge and perspective of heterogeneous photocatalysts. Chem Soc Rev 42:2568–2580
Zhang XR, Guo YS, Liu MS, Zhang SS (2013) Photoelectrochemically active species and photoelectrochemical biosensors. RSC Adv 3:2846–2857
Zhang N, Zhang L, Ruan YF, Zhao WW, Xu JJ, Chen HY (2017) Quantum-dots-based photoelectrochemical bioanalysis highlighted with recent examples. Biosens Bioelectron 94:207–218
Gill R, Zayats M, Willner I (2008) Semiconductor quantum dots for bioanalysis. Angew Chem 47:7602–7625
Yan K, Wang R, Zhang JD (2014) A photoelectrochemical biosensor for o-aminophenol based on assembling of CdSe and DNA on TiO2 film electrode. Biosens Bioelectron 53:301–304
Zhao WW, Shan S, Ma ZY, Wan LN, Xu JJ, Chen HY (2013) Acetylcholine esterase antibodies on BiOI nanoflakes/TiO2 nanoparticles electrode: a case of application for general photoelectrochemical enzymatic analysis. Anal Chem 85:11686–11690
Wang GL, Liu KL, Dong YM, Wu XM, Li ZJ, Zhang C (2014) A new approach to light up the application of semiconductor nanomaterials for photoelectrochemical biosensors: using self-operating photocathode as a highly selective enzyme sensor. Biosens Bioelectron 62:66–72
Wang GL, Shu JX, Dong YM, Wu XM, Zhao WW, Xu JJ, Chen HY (2015) Using G-quadruplex/hemin to "switch-on" the cathodic photocurrent of p-type PbS quantum dots: toward a versatile platform for photoelectrochemical aptasensing. Anal Chem 87:2892–2900
Li Y, Chen FT, Luan ZZ, Zhang XR (2018) A versatile cathodic "signal-on" photoelectrochemical platform based on a dual-signal amplification strategy. Biosens Bioelectron 119:63–69
Zhao WW, Dong XY, Wang J, Kong FY, Xu JJ, Chen HY (2012) Immunogold labeling-induced synergy effect for amplified photoelectrochemical immunoassay of prostate-specific antigen. Chem Commun 48:5253–5255
Kong C, Zhang G, Li Y, Li DW, Long YT (2013) Plasmon-enhanced photocurrent monitoring of the interaction between porphyrin covalently bonded to graphene oxide and adenosine nucleotides. RSC Adv 3:3503–3507
Fan CC, Shi XM, Zhang JR, Zhu JJ (2016) Cathode photoelectrochemical immunosensing platform integrating photocathode with photoanode. Anal Chem 88:10352–10356
Song Z, Fan GC, Li ZM, Gao FX, Luo XL (2018) Universal design of selectivity-enhanced photoelectrochemical enzyme sensor: integrating photoanode with biocathode. Anal Chem 90:10681–10687
Jiang XY, Zhang L, Liu YL, Yu XD, Liang YY, Qu P, Zhao WW, Xu JJ, Chen HY (2018) Hierarchical CuInS2-based heterostructure: application for photocathodic bioanalysis of sarcosine. Biosens Bioelectron 107:230–236
Zheng YN, Liang WB, Xiong CY, Yuan YL, Chai YQ, Yuan R (2016) Self-enhanced ultrasensitive photoelectrochemical biosensor based on nanocapsule packaging both donor-acceptor-type photoactive material and its sensitizer. Anal Chem 88:8698–8705
Zhao WW, Ma ZY, Yu PP, Dong XY, Xu JJ, Chen HY (2012) Highly sensitive photoelectrochemical immunoassay with enhanced amplification using horseradish peroxidase induced biocatalytic precipitation on a CdS quantum dots multilayer electrode. Anal Chem 84:917–923
Shen QM, Han L, Fan GH, Zhang JR, Jiang L, Zhu JJ (2015) “Signal-on” photoelectrochemical biosensor for sensitive detection of human T-cell lymphotropic virus type II DNA: dual signal amplification strategy integrating enzymatic amplification with terminal deoxynucleotidyl transferase-mediated extension. Anal Chem 87:4949–4956
Li MJ, Zheng YN, Liang WB, Yuan R, Chai YQ (2017) Using p-type PbS quantum dots to quench photocurrent of fullerene−AuNP@MoS2composite structure for ultrasensitive photoelectrochemical detection of ATP. ACS Appl Mater Interfaces 9:42111–44212
Zhang XR, Xu YP, Zhao YQ, Song W (2013) A new photoelectrochemical biosensors based on DNA conformational changes and isothermal circular strand-displacement polymerization reaction. Biosens Bioelectron 39:338–341
Shi XM, Fan GG, Shen QM, Zhu JJ (2016) Photoelectrochemical DNA biosensor based on dual-signal amplification strategy integrating inorganic−organic nanocomposites sensitization with λ-exonuclease-assisted target recycling. ACS Appl Mater Interfaces 83:5091–35098
Ma ZY, Ruan YF, Zhang N, Zhao WW, Xu JJ, Chen HY (2015) A new visible-light-driven photoelectrochemical biosensor for probing DNA–protein interactions. Chem Commun 51:8381–8384
Ma ZY, Ruan YF, Xu F, Zhao WW, Xu JJ, Chen HY (2016) Protein binding bends the gold nanoparticle capped DNA sequence: toward novel energy-transfer-based photoelectrochemical protein detection. Anal Chem 88:3864–3871
Oudeng G, Au M, Shi JY, Wen CY, Yang M (2018) One-step in situ detection of miRNA-21 expression in single cancer cells based on biofunctionalized MoS2 nanosheets. ACS Appl Mater Interfaces 10:350–360
Fang CS, Kim KS, Yu B, Jon S, Kim MS, Yang H (2017) Ultrasensitive electrochemical detection of miRNA-21 using a zinc finger protein specific to DNA-RNA hybrids. Anal Chem 89:2024–2031
Wang JX, Zhang LL, Lu LP, Kang TF (2019) Molecular beacon immobilized on graphene oxide for enzyme-free signal amplification in electrochemiluminescent determination of microRNA. Microchim Acta 186:2563–2569
Mahani M, Mousapour Z, Divsar F, Nomani A, Ju HX (2019) A carbon dot and molecular beacon based fluorometric sensor for the cancer marker microRNA-21. Microchim Acta 186:132
Tang YF, Liu MX, Zhao ZL, Li Q, Liang XH, Tian JN, Zhao SL (2019) Fluorometric determination of microRNA-122 by using ExoIII-aided recycling amplification and polythymine induced formation of copper nanoparticles. Microchim Acta 186:133
Liu NN, Yang ZK, Lou XD, Wei BM, Zhang JT, Gao PC, Hou RZ, Xia X (2015) Nanopore-based DNA-probe sequence-evolution method unveiling characteristics of protein-DNA binding phenomena in a nanoscale confined space. Anal Chem 87:4037–4041
Liao H, Zhou Y, Chai Y, Yuan R (2018) An ultrasensitive electrochemiluminescence biosensor for detection of MicroRNA by in-situ electrochemically generated copper nanoclusters as luminophore and TiO2 as coreaction accelerator. Biosens Bioelectron 114:10–14
Tang Y, Liu M, Xu L, Tian J, Yang X, Zhao Y, Zhao S (2018) A simple and rapid dual-cycle amplification strategy for microRNA based on graphene oxide and exonuclease III-assisted fluorescence recovery. Anal Methods 10:3777–3782
Huang RC, Chiu WJ, Li YJ, Huang CC (2014) Detection of microRNA in tumor cells using exonuclease III and graphene oxide-regulated signal amplification. ACS Appl Mater Interfaces 6:21780–21787
Min X, Zhang M, Huang F, Lou X, Xia F (2016) Live cell MicroRNA imaging using exonuclease III-aided recycling amplification based on aggregation-induced emission Luminogens. ACS Appl Mater Interfaces 8:8998–9003
Chen A, Gui GF, Zhuo Y, Chai YQ, Xiang Y, Yuan R (2015) Signal-off electrochemiluminescence biosensor based on Phi29 DNA polymerase mediated strand displacement amplification for microRNA detection. Anal Chem 87:6328–6334
Liao YH, Huang R, Ma ZK, Wu YX, Zhou XM, Xing D (2014) Target-triggered enzyme-free amplification strategy for sensitive detection of MicroRNA in tumor cells and tissues. Anal Chem 86:4596–4604
Yin BC, Liu YQ, Ye BC (2013) Sensitive detection of microRNA in complex biological samples via enzymatic signal amplification using DNA polymerase coupled with nicking endonuclease. Anal Chem 85:1487–11493
Lin MH, Wen YL, Li LY, Pei H, Liu G, Song HY, Zuo XL, Fan CH, Huang Q (2014) Target-responsive, DNA nanostructure-based E-DNA sensor for microRNA analysis. Anal Chem 86:2285–2288
Liu WP, Zhou XM, Xing D (2014) Rapid and reliable microRNA detection by stacking hybridization on electrochemiluminescent chip system. Biosens Bioelectron 58:388–394
Duan RX, Zuo XL, Wang ST, Quan XY, Chen DL, Chen ZF, Jiang L, Fan CH, Xia F (2013) Lab in a tube: ultrasensitive detection of MicroRNAs at the single-cell level and in breast cancer patients using quadratic isothermal amplification. J Am Chem Soc 135:4604–4607
Labib M, Khan N, Ghobadloo SM, Cheng J, Pezacki JP, Berezovski MV (2013) Three-mode electrochemical sensing of ultralow MicroRNA levels. J Am Chem Soc 135:3027–3038
Cong XX, Zhou MF, Hou T, Xu ZJ, Yin YZ, Wang XL, Yin M (2018) A sensitive photoelectrochemical aptasensor or miRNA-21 based on the sensitization effect of CdSe quantum dots. Electroanalysis 30:1140–1146
Wang B, Dong YX, Wang YL, Cao JT, Ma SH, Liu YM (2018) Quenching effect of exciton energy transfer from CdS:Mn to Au nanoparticles: a highly effcient photoelectrochemical strategy for microRNA-21 detection. Sensors Actuators B Chem 254:159–165
Acknowledgements
This work has been financially supported by the National Natural Science Foundation of China (Nos.: 21775080, 21705086); Key Research and Development Project of Shandong Province, China (No. 2017GSF221004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 717 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Zhao, L., Liang, D. et al. An CuInS2 photocathode for the sensitive photoelectrochemical determination of microRNA-21 based on DNA–protein interaction and exonuclease III assisted target recycling amplification. Microchim Acta 186, 692 (2019). https://doi.org/10.1007/s00604-019-3804-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3804-z