Abstract
Carboxylic acids (CAs) have been reported as potential biomarkers of specific diseases or human body odors. A visual sensor array is described here that is based on indicator displacement assays (IDAs). The arrays were prepared by spotting solutions of the following metal complexes: Murexide-Ni(II), murexide-Cu(II), zincon-Zn(II) and xylenol orange-Cu(II), with the capability of discrimination of 15 carboxylic acids (CAs) and the quantitation of pyruvic acid (PA). Clear differences can be observed through distinctive difference maps obtained within 5 min by subtraction of red, green and blue (RGB) values of digital images after and before exposure to analytes. After an analysis of multidimensional data by pattern recognition algorithms including HCA, PCA and LDA, excellent classification specificity, and accuracy of >96% were obtained for all samples. The IDA array exhibited a linear range from 10 to 1500 μM with a theoretical detection limit of 3.5 μM towards PA. Recoveries of real samples varied from 84.8% to 114.3%. As-fabricated IDA sensor array showed an excellent selectivity among other organic interfering substances and a good batch to batch reproducibility, demonstrating its robustness. All these observations suggested that the IDA sensor array is one of the most promising paths for the discrimination of CAs.

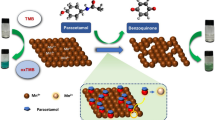
Schematic diagram of indicator displacement assay (a), the procedure for acquisition of difference maps (b), and pattern recognitions for CAs (c). The method uses hierarchical cluster analysis (HCA), principal component analysis (PCA) and linear discriminant analysis (LDA)








Similar content being viewed by others
References
Lehotay DC (2010) Chromatographic techniques in inborn errors of metabolism. Biomed Chromatogr 5(3):113–121
Kolk AH, van Berkel JJ, Claassens MM, Walters E, Kuijper S, Dallinga JW, van Schooten FJ (2012) Breath analysis as a potential diagnostic tool for tuberculosis. Int J Tuberc Lung Dis 16(6):777–782
Zhong X, Li D, Du W, Yan M, Wang Y, Huo D, Hou C (2018) Rapid recognition of volatile organic compounds with colorimetric sensor arrays for lung cancer screening. Anal Bioanal Chem 410(16):3671–3681
Shang L, Liu C, Watanabe M, Chen B, Hayashi K (2017) LSPR sensor array based on molecularly imprinted sol-gels for pattern recognition of volatile organic acids. Sensors Actuators B Chem 249:14–21
Natsch A, Derrer S, Flachsmann F, Schmid J (2010) A broad diversity of volatile carboxylic acids, released by a bacterial Aminoacylase from axilla secretions, as candidate molecules for the determination of human-body odor type. Chem Biodivers 3(1):1–20
Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, Preti G (2008) Analyses of volatile organic compounds from human skin. Br J Dermatol 159(4):780–791
Mochalski P, Unterkofler K, Teschl G, Amann A (2015) Potential of volatile organic compounds as markers of entrapped humans for use in urban search-and-rescue operations. TrAC Trends Anal Chem 68:88–106
Kładna A, Marchlewicz M, Piechowska T, Kruk I, Aboul-Enein HY (2015) Reactivity of pyruvic acid and its derivatives towards reactive oxygen species. Luminescence 30(7):1153–1158
Brahman PK, Pandey N, Topkaya SN, Singhai R (2015) Fullerene-C60-MWCNT composite film based ultrasensitive electrochemical sensing platform for the trace analysis of pyruvic acid in biological fluids. Talanta 134:554–559
Stefan-van Staden R-I, Diaconeasa AG, Gugoasa LA, Rosu M-C, Pruneanu S (2017) Molecular recognition of pyruvic acid and folic acid in whole blood. RSC Adv 7(79):50072–50078
Ahmad M, Gazala Mohamed BH, Bahruddin S (2015) Determination of α-ketoglutaric and pyruvic acids in urine as potential biomarkers for diabetic II and liver cancer. Bioanalysis 7(6):713–723
Curran AM, Rabin SI, Prada PA, Furton KG (2005) Comparison of the volatile organic compounds present in human odor using Spme-GC/MS. J Chem Ecol 31(7):1607–1619
Tang Z, Duan Y (2017) Fabrication of porous ionic liquid polymer as solid-phase microextraction coating for analysis of organic acids by gas chromatography - mass spectrometry. Talanta 172:45–52
Liu C, Furusawa Y, Hayashi K (2013) Development of a fluorescent imaging sensor for the detection of human body sweat odor. Sensors Actuators B Chem 183:117–123
Davey EA, Zucchero AJ, Trapp O, Bunz UH (2011) Discrimination of organic acids using a three molecule array based upon cruciform fluorophores. J Am Chem Soc 133(20):7716–7718
Han J, Bender M, Hahn S, Seehafer K, Bunz UH (2016) Polyelectrolyte complexes formed from conjugated polymers: Array-based sensing of organic acids. Chemistry 22(10):3230–3233
Jha SK, Liu C, Hayashi K (2014) Molecular imprinted polyacrylic acids based QCM sensor array for recognition of organic acids in body odor. Sensors Actuators B Chem 204:74–87
Liu C, Shang L, Yoshioka HT, Chen B, Hayashi K (2018) Preparation of molecularly imprinted polymer nanobeads for selective sensing of carboxylic acid vapors. Anal Chim Acta 1010:1–10
Baliyan A, Bhatia P, Gupta BD, Sharma EK, Kumari A, Gupta R (2013) Surface plasmon resonance based fiber optic sensor for the detection of triacylglycerides using gel entrapment technique. Sensors Actuators B Chem 188:917–922
Kang J, Hussain AT, Catt M, Trenell M, Haggett B, Yu EH (2014) Electrochemical detection of non-esterified fatty acid by layer-by-layer assembled enzyme electrodes. Sensors Actuators B Chem 190:535–541
Askim JR, Mahmoudi M, Suslick KS (2013) Optical sensor arrays for chemical sensing: the optoelectronic nose. Chem Soc Rev 42(22):8649–8682
Torsten M, Christian I, Gregor L, Ingo K, Wolfbeis OS (2003) Cross-reactive metal ion sensor array in a micro titer plate format. Anal Chem 75(17):4389–4396
Rakow NA, Sen A, Janzen MC, Ponder JB, Suslick KS (2005) Molecular recognition and discrimination of amines with a colorimetric array. Angew Chem 44(29):4528–4532
Feng L, Li H, Li X, Chen L, Shen Z, Guan Y (2012) Colorimetric sensing of anions in water using ratiometric indicator-displacement assay. Anal Chim Acta 743:1–8
And AB, Severin K (2005) A Chemosensor Array for the colorimetric identification of 20 natural amino acids. J Am Chem Soc 127(11):3700–3701
Khajehsharifi H, Sheini A (2014) A selective naked-eye detection and determination of cysteine using an indicator-displacement assay in urine sample. Sensors Actuators B Chem 199:457–462
Zhang D (2009) Highly selective colorimetric detection of cysteine and homocysteine in water through a direct displacement approach. Inorg Chem Commun 12(12):1255–1258
Umali AP, LeBoeuf SE, Newberry RW, Kim S, Tran L, Rome WA, Tian T, Taing D, Hong J, Kwan M, Heymann H, Anslyn EV (2011) Discrimination of flavonoids and red wine varietals by arrays of differential peptidic sensors. Chem Sci 2(3):439–445
Zhang X, Anslyn EV, Qian X (2012) Discrimination of vicinal-diol-containing flavonoids and black teas by arrays of host–indicator ensembles. Supramol Chem 24(7):520–525
Sheini A, Khajehsharifi H, Shahbazy M, Kompany-Zareh M (2017) A chemosensor array for the colorimetric identification of some carboxylic acids in human urine samples. Sensors Actuators B Chem 242:288–298
Cornard JP, Boudet AC, Merlin JC (2001) Complexes of Al(III) with 3′4′-dihydroxy-flavone: characterization, theoretical and spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc 57(3):591–602
Zhong X, Huo D, Fa H, Luo X, Wang Y, Zhao Y, Hou C (2018) Rapid and ultrasensitive detection of biogenic amines with colorimetric sensor array. Sensors Actuators B Chem 274:464–471
Wang J, Peng D (2011) Direct electrochemical detection of pyruvic acid by cobalt oxyhydroxide modified indium tin oxide electrodes. Electrochim Acta 56(27):10159–10165
Canbay E, Habip A, Kara G, Eren Z, Akyilmaz E (2015) A microbial biosensor based on Lactobacillus delbruecki sp. bacterial cells for simultaneous determination of lactic and pyruvic acid. Food Chem 169:197–202
Ghica ME, Brett CMA (2006) Development of novel glucose and pyruvate biosensors at poly(neutral red) modified carbon film electrodes. Application to natural samples. Electroanalysis 18(8):748–756
Li W, Pan C, Hou T, Wang X, Li F (2014) Selective and colorimetric detection of pyruvic acid using conformational switch of i-motif DNA and unmodified gold nanoparticles. Anal Methods 6(6):1645–1649
Metrani R, Jayaprakasha GK, Patil BS (2018) Optimized method for the quantification of pyruvic acid in onions by microplate reader and confirmation by high resolution mass spectra. Food Chem 242:451–458
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (NSFC) (No.81772290, 81271930), Graduate research and innovation foundation of Chongqing, China (Grant No. CYB18025), and Brew Microorganisms Technology and Application of Key Laboratory Project in Sichuan Province (No. NJ2018-01) and the sharing fund of Chongqing University’s large equipment for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 6.08 mb)
Rights and permissions
About this article
Cite this article
Wang, Y., Huo, D., Wu, H. et al. A visual sensor array based on an indicator displacement assay for the detection of carboxylic acids. Microchim Acta 186, 496 (2019). https://doi.org/10.1007/s00604-019-3601-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3601-8