Abstract
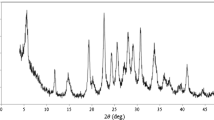
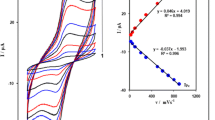
β-Nickel hydroxide nanoplatelets (NPs) were synthesized and used as a modifier on a carbon paste electrode (CPE) for the detection of hydrazine. Synthesis is based on a one-step hydrothermal method using L-arginine that acts as an agent to adjust the pH value and to shape the NPs. They were characterized by field emission scanning electron microscopy, X-ray diffraction and energy-dispersive X-ray spectroscopy. The composition of the modified electrode was optimized by changing the amount of NPs. Best results were achieved using a 10:70:20 weight ratio for nanoparticles, graphite and mineral oil (the binder). The electrochemical properties of the modified CPE were studied by cyclic voltammetry. The surface coverage of the NPs, the electron transfer coefficient and the charge transfer rate constant were calculated. The diffusion coefficient of hydrazine is 1.56 × 10−5 cm2 s−1. An amperometric method was worked out that has the following figures of merit: (a) A working applied potential of 500 mV (vs. Ag/AgCl reference electrode), (b) a linear range that extends from 1 to 1300 μmol L−1, (c) a 0.28 μmol L−1 detection limit, (d) a relative standard deviation of 1.3% (for n = 3 at a level of 5 μmol L−1), and (d) a sensitivity of 1.33 μA μmol−1 L cm−2. The performance of the sensor is compared to other nickel-based sensors for hydrazine. The sensor was successfully used to quantify hydrazine in spiked tap water samples, and the recoveries were 97 ± 2.3% (n = 3).

Schematic presentation of hydrazine electrocatalytic oxidation on NP.CPE*. *NP-CPE (β-nickel hydroxide nanoplatelet modified carbon paste electrode)






Similar content being viewed by others
Change history
09 July 2019
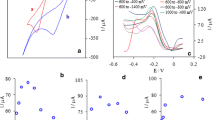
The authors of “Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with β-nickel hydroxide nanoplatelets (Microchimica Acta (2019) 186:441)” wish to update the incorrect panel/labels of Figure 3.
References
George M, Nagaraja KS, Balasubramanian N (2008) Spectrophotometric determination of hydrazine. Talanta 75(1):27–31. https://doi.org/10.1016/j.talanta.2007.09.002
Selim S, Warner CR (1978) Residue determination of hydrazine in water by derivatization and gas chromatography. J Chromatogr A 166(2):507–511. https://doi.org/10.1016/S0021-9673(00)95634-6
Oh J-A, Park J-H, Shin H-S (2013) Sensitive determination of hydrazine in water by gas chromatography–mass spectrometry after derivatization with ortho-phthalaldehyde. Anal Chim Acta 769:79–83. https://doi.org/10.1016/j.aca.2013.01.036
Smolenkov AD, Shpigun OA (2012) Direct liquid chromatographic determination of hydrazines: a review. Talanta 102:93–100
Hamidi H, Bozorgzadeh S, Haghighi B (2017) Amperometric hydrazine sensor using a glassy carbon electrode modified with gold nanoparticle-decorated multiwalled carbon nanotubes. Microchim Acta 184(11):4537–4543. https://doi.org/10.1007/s00604-017-2480-0
Rees NV, Compton RG (2011) Carbon-free energy: a review of ammonia-and hydrazine-based electrochemical fuel cells. Energy Environ Sci 4(4):1255–1260
Serov A, Kwak C (2010) Direct hydrazine fuel cells: a review. Appl Catal B 98:1):1–1):9. https://doi.org/10.1016/j.apcatb.2010.05.005
Miao Y, Ouyang L, Zhou S, Xu L, Yang Z, Xiao M, Ouyang R (2014) Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosens Bioelectron 53:428–439
Zhu X, Zhong Y, Zhai H, Yan Z, Li D (2014) Nanoflake nickel hydroxide and reduced graphene oxide composite as anode materials for high capacity lithium ion batteries. Electrochim Acta 132:364–369. https://doi.org/10.1016/j.electacta.2014.03.132
Lakshmi V, Ranjusha R, Vineeth S, Nair SV, Balakrishnan A (2014) Supercapacitors based on microporous β-Ni(OH)2 nanorods. Colloids Surf A Physicochem Eng Asp 457:462–468. https://doi.org/10.1016/j.colsurfa.2014.06.016
Wang D, Yan W, Vijapur SH, Botte GG (2012) Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J Power Sources 217:498–502. https://doi.org/10.1016/j.jpowsour.2012.06.029
Qiao NQ, Zheng JB (2012) Nonenzymatic glucose sensor based on glassy carbon electrode modified with a nanocomposite composed of nickel hydroxide and graphene. Microchim Acta 177(1–2):103–109. https://doi.org/10.1007/s00604-011-0756-3
Martínez-Periñán E, Revenga-Parra M, Gennari M, Pariente F, Mas-Ballesté R, Zamora F, Lorenzo E (2016) Insulin sensor based on nanoparticle-decorated multiwalled carbon nanotubes modified electrodes. Sens Actuators B 222:331–338. https://doi.org/10.1016/j.snb.2015.08.033
Chen Z, Nai J, Ma H, Li Z (2014) Nickel hydroxide nanocrystals-modified glassy carbon electrodes for sensitive l-histidine detection. Electrochim Acta 116:258–262. https://doi.org/10.1016/j.electacta.2013.10.153
Babaei A, Yousefi A, Afrasiabi M, Shabanian M (2015) A sensitive simultaneous determination of dopamine, acetaminophen and indomethacin on a glassy carbon electrode coated with a new composite of MCM-41 molecular sieve/nickel hydroxide nanoparticles/multiwalled carbon nanotubes. J Electroanal Chem 740:28–36. https://doi.org/10.1016/j.jelechem.2014.12.042
Zhang X, Huang Y, Gu A, Wang G, Fang B, Wu H (2012) Hydrogen peroxide sensor based on carbon nanotubes/β-Ni (OH) 2 nanocomposites. Chin J Chem 30(3):501–506
Heli H, Sattarahmady N, Vais RD, Karimian K (2014) Nickel hydroxide nanopetals: one-pot green synthesis, characterization and application for the electrocatalytic oxidation and sensitive detection of montelukast. Sensors Actuators B 196:631–639. https://doi.org/10.1016/j.snb.2014.02.057
Hajjizadeh M, Jabbari A, Heli H, Moosavi-Movahedi AA, Haghgoo S (2007) Electrocatalytic oxidation of some anti-inflammatory drugs on a nickel hydroxide-modified nickel electrode. Electrochim Acta 53(4):1766–1774. https://doi.org/10.1016/j.electacta.2007.08.026
Nesterov B, Korovin N (1966) Anodic oxidation of hydrazine on smooth nickel in alkaline solution. Elektrokhimiya 2(11):1296
Sakamoto T, Asazawa K, Sanabria-Chinchilla J, Martinez U, Halevi B, Atanassov P, Strasser P, Tanaka H (2014) Combinatorial discovery of Ni-based binary and ternary catalysts for hydrazine electrooxidation for use in anion exchange membrane fuel cells. J Power Sources 247:605–611. https://doi.org/10.1016/j.jpowsour.2013.08.107
Abbaspour A, Khajehzadeh A, Ghaffarinejad A (2009) Electrocatalytic oxidation and determination of hydrazine on nickel hexacyanoferrate nanoparticles-modified carbon ceramic electrode. J Electroanal Chem 631(1–2):52–57. https://doi.org/10.1016/j.jelechem.2009.03.011
Adekunle AS, Ozoemena KI (2010) Electron transport and electrocatalytic properties of MWCNT/nickel nanocomposites: hydrazine and diethylaminoethanethiol as analytical probes. J Electroanal Chem 645(1):41–49. https://doi.org/10.1016/j.jelechem.2010.04.010
Azad UP, Ganesan V (2011) Determination of hydrazine by polyNi(II) complex modified electrodes with a wide linear calibration range. Electrochim Acta 56(16):5766–5770. https://doi.org/10.1016/j.electacta.2011.04.051
Jeevagan AJ, John SA (2013) Synthesis of non-peripheral amine substituted nickel(ii) phthalocyanine capped gold nanoparticles and their immobilization on electrode for the electrocatalytic oxidation of hydrazine. RSC Adv 3(7):2256–2264. https://doi.org/10.1039/c2ra22895e
Kazemi SH, Hosseinzadeh B, Zakavi S (2015) Electrochemical fabrication of conducting polymer of Ni-porphyrin as nano-structured electrocatalyst for hydrazine oxidation. Sensors Actuators B Chem 210:343–348. https://doi.org/10.1016/j.snb.2014.12.131
Pandey PC, Panday D (2016) Novel synthesis of nickel–iron hexacyanoferrate nanoparticles and its application in electrochemical sensing. J Electroanal Chem 763:63–70. https://doi.org/10.1016/j.jelechem.2015.12.048
Zheng L, J-f S (2009) Ni(II)–baicalein complex modified multi-wall carbon nanotube paste electrode toward electrocatalytic oxidation of hydrazine. Talanta 79(2):319–326. https://doi.org/10.1016/j.talanta.2009.03.056
Ji R, Huang Y, Wang L, Yu L, Wang J, Wang G, Zhang X (2013) Synthesis of Ni(OH)2 nanoplates on cu rod and its applications for electrochemical sensors. Mater Res Bull 48(10):3729–3734. https://doi.org/10.1016/j.materresbull.2013.05.115
Anu Prathap MU, Anuraj V, Satpati B, Srivastava R (2013) Facile preparation of Ni(OH)2–MnO2 hybrid material and its application in the electrocatalytic oxidation of hydrazine. J Hazard Mater 262:766–774. https://doi.org/10.1016/j.jhazmat.2013.09.050
Anthony J, Bideaux R, Bladh K, Nichols M (1997) Handbook of mineralogy, halides, hydroxides, oxides, Vol. III. Mineral Data Pub
Bard AJ, Faulkner LR (eds) (2001) Electrochemical methods, fundamentals and applications, 6th edn. Wiley, New York
Sattarahmady N, Heli H, Faramarzi F (2010) Nickel oxide nanotubes-carbon microparticles/Nafion nanocomposite for the electrooxidation and sensitive detection of metformin. Talanta 82(4):1126–1135. https://doi.org/10.1016/j.talanta.2010.06.022
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem 101(1):19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Karp S, Meites L (1962) The Voltammetric characteristics and mechanism of Electrooxidation of hydrazine. J Am Chem Soc 84(6):906–912. https://doi.org/10.1021/ja00865a006
Acknowledgements
The authors wish to express their thanks to the research council of university of Maragheh for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Unfortunately in the online version of the article, Fig. 3 has four parts which should be labeled as a, b, c, and d; but incorrectly labeled as a, b, d, and d. There is two “d” in and no “c”. The correct image is given in this article.
Electronic supplementary material
ESM 1
(DOCX 260 kb)
Rights and permissions
About this article
Cite this article
Avanes, A., Hasanzadeh-Karamjavan, M. & Shokri-Jarcheloo, G. Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with β-nickel hydroxide nanoplatelets. Microchim Acta 186, 441 (2019). https://doi.org/10.1007/s00604-019-3555-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3555-x