Abstract
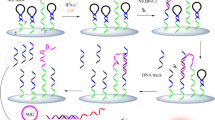
A strategy is described for continuous monitoring of multiple latent tuberculosis infection (LTBI) biomarkers, specifically of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and interleukin-2 (IL-2). Silver nanoparticles acting as mass signal amplifiers were linked to respective antibodies to form mass nanoprobes for increasing the mass loaded on the surface of the quartz crystal microbalance (QCM). This results in enhanced sensitivity. The mass nanoprobes can be oxidatively dissolved by hydrogen peroxide that avoided the steric hindrance caused by the scale effect of mass nanoprobes. This offers the option of signal recovery monitoring. By using this method, IFN-γ, TNF-α and IL-2 can be monitored serially. The frequency shifts caused by TNF-α, IFN-γ and IL-2, respectively, are reversible. Hence, the biomarkers can be continuously quantified. Compared to multichannel QCM sensing, the new method avoids acoustic interference and has a simplified instrumental setup. The assay is simple, accurate, sensitive, and inexpensive.

Silver nanoparticles as the mass signal amplifiers were linked with the antibodies to form mass nanoprobes for enhancing the monitoring sensitivity. With the introduction of H2O2 to dissolve the mass nanoprobes attached on sensing interface, a signal recovery QCM strategy is established for real-time and continuous monitoring of three LTBI biomarkers.





Similar content being viewed by others
References
Lönnroth K, Migliori GB, Abubakar I, D'Ambrosio L, de Vries G, Diel R, Douglas P, Falzon D, Gaudreau MA, Goletti D et al (2015) Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 45:928–952
Zumla A, Chakaya J, Centis R, D'Ambrosio L, Mwaba P, Bates M, Kapata N, Nyirenda T, Chanda D, Mfinanga S, Hoelscher M, Maeurer M, Migliori GB (2015) Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 3(3):220–234
Pai M, Dheda K, Cunningham J, Scano F, O'Brien R (2007) T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis 7(6):428–438
Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG (2007) Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 175(1):87–93
Lange C, Pai M, Drobniewski F, Migliori GB (2009) Interferon-γ release assays for the diagnosis of active tuberculosis: sensible or silly? Eur Respir J 33:1250–1253
Prabhavathi M, Pathakumari B, Raja A (2015) IFN-gamma/TNF-alpha ratio in response to immuno proteomically identified human T-cell antigens of mycobacterium tuberculosis - the most suitable surrogate biomarker for latent TB infection. J Inf Secur 71(2):238–249
Wu J, Wang S, Lu C, Shao L, Gao Y, Zhou Z, Huang H, Zhang Y, Zhang W (2017) Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis 102:68–75
Lucas M, Nicol P, McKinnon E, Whidborne R, Lucas A, Thambiran A, Burgner D, Waring J, French M (2010) A prospective large-scale study of methods for the detection of latent mycobacterium tuberculosis infection in refugee children. Thorax 65:442–448
Pai M, Riley LW, Colford JM Jr (2004) Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 4(12):761–776
Johnson JL, Ssekasanvu E, Okwera A, Mayanja H, Hirsch CS, Nakibali JG, Jankus DD, Eisenach KD, Boom WH, Ellner JJ, Mugerwa RD (2003) Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med 168(2):185–191
Schauf V, Rom WN, Smith KA, Sampaio EP, Meyn PA, Tramontana JM, Cohn ZA, Kaplan G (1993) Cytokine gene activation and modified responsiveness to interleukin-2 in the blood of tuberculosis patients. J Infect Dis 168(4):1056–1059
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M et al (2004) Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 170(1):59–64
Chen Y, Pui TS, Kongsuphol P, Tang KC, Arya SK (2014) Aptamer-based array electrodes for quantitative interferon-gamma detection. Biosens Bioelectron 53:257–262
Zhang Y, Zhang B, Ye X, Yan Y, Huang L, Jiang Z, Tan S, Cai X (2016) Electrochemical immunosensor for interferon-gamma based on disposable ITO detector and HRP-antibody-conjugated nano gold as signal tag. Mater Sci Eng, C 59:577–584
Zhou B, Zhu M, Qiu Y, Yang P (2017) Novel electrochemiluminescence-sensing platform for the precise analysis of multiple latent tuberculosis infection markers. ACS Appl Mater Interfaces 9(22):18493–18500
Zhou B, Zhu M, Hao Y, Yang P (2017) Potential-resolved electrochemiluminescence for simultaneous determination of triple latent tuberculosis infection markers. ACS Appl Mater Interfaces 9(36):30536–30542
Escalante P, Peikert T, Van Keulen VP, Erskine CL, Bornhorst CL, Andrist BR, McCoy K, Pease LR, Abraham RS, Knutson KL, Kita H, Schrum AG, Limper AH (2015) Combinatorial immunoprofiling in latent tuberculosis infection. Toward better risk stratification. Am J Respir Crit Care Med 192(5):605–617
Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, Day CL, Ray SM, Rengarajan J (2015) Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest 125(5):1827–1838
Olsson AL, Wargenau A, Tufenkji N (2016) Optimizing bacteriophage surface densities for bacterial capture and sensing in quartz crystal microbalance with dissipation monitoring. ACS Appl Mater Interfaces 8(22):13698–13706
Wang RH, Wang LJ, Callaway ZT, Lu HG, Huang TJ, Li YB (2017) A nanowell-based QCM aptasensor for rapid and sensitive detection of avian influenza virus. Sensors Actuators B Chem 240:934–940
Chunta S, Suedee R, Lieberzeit PA (2015) Low-density lipoprotein sensor based on molecularly imprinted polymer. Anal Chem 88:1419–1425
Sun WB, Song WL, Guo XY, Wang ZH (2017) Ultrasensitive detection of nucleic acids and proteins using quartz crystal microbalance and surface plasmon resonance sensors based on target-triggering multiple signal amplification strategy. Anal Chim Acta 978:42–47
Su JW, Esmaeilzadeh H, Zhang F, Yu Q, Cernigliaro G, Xu J, Sun HW (2018) An ultrasensitive micropillar-based quartz crystal microbalance device for real-time measurement of protein immobilization and protein-protein interaction. Biosens Bioelectron 99:325–331
Minsky BB, Antoni CH, Boehm H (2016) Controlled immobilization strategies to probe short hyaluronan-protein interactions. Sci Rep 6:21608
Azuma T, Teramura Y, Takai M (2016) Cellular response to non-contacting nanoscale sublayer: cells sense several nanometer mechanical property. ACS Appl Mater Interfaces 8(17):10710–10716
Chen JY, Penn LS, Xi J (2018) Quartz crystal microbalance: sensing cell-substrate adhesion and beyond. Biosens Bioelectron 99:593–602
Cooper MA, Singleton VT (2007) A survey of the 2001 to 2005 quartz crystal microbalance biosensor literature: applications of acoustic physics to the analysis of biomolecular interactions. J Mol Recognit 20(3):154–184
Deng XD, Chen MS, Fu Q, Smeets NMB, Xu F, Zhang ZY, Filipe CDM, Hoare T (2016) A highly sensitive immunosorbent assay based on biotinylated graphene oxide and the quartz crystal microbalance. ACS Appl Mater Interfaces 8(3):1893–1902
Tuantranont A, Wisitsora-at A, Sritongkham P, Jaruwongrungsee K (2011) A review of monolithic multichannel quartz crystal microbalance: a review. Anal Chim Acta 687(2):114–128
Jaruwongrungsee K, Waiwijit U, Wisitsoraat A, Sangworasil M, Pintavirooj C, Tuantranont A (2015) Real-time multianalyte biosensors based on interference-free multichannel monolithic quartz crystal microbalance. Biosens Bioelectron 67:576–581
Liang JX, Zhang J, Zhou WX, Ueda T (2017) Development of a flow injection based high frequency dual channel quartz crystal microbalance. Sensors 17(5):1136
Zhou B, Hao Y, Long D, Yang P (2018) Real-time quartz crystal microbalance cytosensor based on a signal recovery strategy for in-situ and continuous monitoring of multiple cell membrane glycoproteins. Biosens Bioelectron 111:90–96
Nishino H, Nihira T, Mori T, Okahata Y (2004) Direct monitoring of enzymatic glucan hydrolysis on a 27-MHz quartz-crystal microbalance. J Am Chem Soc 126(8):2264–2265
Zhou L, Lu P, Zhu M, Li B, Yang P, Cai J (2016) Silver nanocluster based sensitivity amplification of a quartz crystal microbalance gene sensor. Microchim Acta 183(2):881–887
Yu RJ, Ma W, Liu XY, Jin HY, Han HX, Wang HY, Tian H, Long YT (2016) Metal-linked immunosorbent assay (MeLISA): the enzyme-free alternative to ELISA for biomarker detection in serum. Theranostics 6(10):1732–1739
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21874057) and the National Natural Science Foundation of China (No. 21375048).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 558 kb)
Rights and permissions
About this article
Cite this article
Zhou, B., Hao, Y., Chen, S. et al. A quartz crystal microbalance modified with antibody-coated silver nanoparticles acting as mass signal amplifiers for real-time monitoring of three latent tuberculosis infection biomarkers. Microchim Acta 186, 212 (2019). https://doi.org/10.1007/s00604-019-3319-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3319-7