Abstract
An immunosensor is described for the voltammetric determination of α-fetoprotein. It is making use of an AuNP-dendrimer conjugate and an ionic liquid. A gold electrode was first modified with chitosan. Then, the AuNP-dendrimer conjugate was covalently immobilized on the electrode. Following this, an ionic liquid was placed on the electrode via formation of a covalent bond between the amino groups of PAMAM and the aldehyde groups of an ionic liquid containing ferrocene. Thus, the redox probe ferrocene becomes immobilized on the electrode surface. PAMAM increases the amount of ferrocene immobilized on the electrode due to its globular shape and rich amino groups. The use of AuNPs improves the conductivity of the electrode. The modified electrode was applied to the determination of α-fetoprotein in human serum and has a linear response that covers the 0.05 to 30 ng mL−1 α-fetoprotein concentration range, with a detection limit of 0.02 ng mL−1. This assay is stable, selective and reproducible. It is perceived to provide a powerful tool for the early detection of cancer markers.

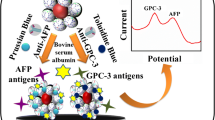
Schematic of a voltammetric immunoassay for α-fetoprotein based on a gold nanoparticle/dendrimer conjugate and ionic liquids anchored with both aldehyde and ferrocene. Chit: chitosan; GA: glutaraldehyde; PAMAM: G4 polyamidoaminic dendrimers; AuNP: Au nanoparticle; Fc: ferrocene; IL: ionic liquid. PB: phosphate buffer solution.





Similar content being viewed by others
References
Xiong C, Wang H, Yuan Y, Chai Y, Yuan R (2015) A novel solid-state Ru(bpy) 3 2+ electrochemiluminescence immunosensor based on poly(ethylenimine) and polyamidoamine dendrimers as co-reactants. Talanta 131:192–197
Akter R, Jeong B, Lee YM, Choi JS, Rahman M (2017) Femtomolar detection of cardiac troponin I using a novel label-free and reagent-free dendrimer enhanced impedimetric immunosensor. Biosens Bioelectron 91:637–643
Tsukruk VV, Rinderspacher F, Bliznyuk VN (1997) Self-assembled multilayer films from dendrimers. Langmuir 13:2171–2176
Kim DM, Rahman M, Do MH, Ban C, Shim YB (2010) An amperometric chloramphenicol immunosensor based on cadmium sulfide nanoparticles modified-dendrimer bonded conducting polymer. Biosens Bioelectron 25:1781–1788
Giannetto M, Mori L, Mori G, Careri M, Mangia A (2011) New amperometric immunosensor with response enhanced by PAMAM-dendrimers linked via self- assembled monolayers for determination of alpha-fetoprotein in human serum. Sensors Actuators B Chem 159:185–192
Dong J, Zhao H, Xu M, Ma Q, Ai S (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem 141:1980–1986
Gao Q, Han J, Ma Z (2013) Polyamidoamine dendrimers-capped carbon dots/au nanocrystal nanocomposites and its application for electrochemical immunosensor. Biosens Bioelectron 49:323–328
Kavosi B, Salimi A, Hallaj R, Amani K (2014) A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens Bioelectron 52:20–28
Erdem A, Congur G, Mese F (2015) PAMAM dendrimer functionalized magnetic particles developed for voltammetric DNA analysis. J Electroanal Chem 741:51–55
Jiang W, Wu L, Duan J, Yin H, Ai S (2018) Ultrasensitive electrochemiluminescence immunosensor for 5-hydroxymethylcytosine detection based on Fe3O4@SiO2 nanoparticles and PAMAM dendrimers. Biosens Bioelectron 99:660–666
Jiang X, Wang H, Yuan R, Chai Y (2015) Sensitive electrochemiluminescence detection for CA15-3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods. Biosens Bioelectron 63:33–38
Kavosi B, Hallaj R, Teymourian H, Salimi A (2014) Au nanoparticles/PAMAM dendrimer functionalized wired ethyleneamine–viologen as highly efficient interface for ultra-sensitive α-fetoprotein electrochemical immunosensor. Biosens Bioelectron 59(2014):389–396
Park S, Kazlauskas RJ (2003) Biocatalysis in ionic liquids-advantages beyond green technology. Curr Opin Biotechnol 14:432–437
Wei Y, Li X, Sun X, Ma H, Zhang Y, Wei Q (2017) Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens Bioelectron 94:141–147
Dong S, Tong M, Zhang D, Huang T (2017) The strategy of nitrite and immunoassay human IgG biosensors based on ZnO@ZIF-8 and ionic liquid composite film. Sensors Actuators B Chem 251:650–657
Fei J, Dou W, Zhao G (2015) A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta 182:2267–2275
Shen G, Zhang X, Shen Y, Zhang S, Fang L (2015) One-step immobilization of antibodies for α-1-fetoprotein immunosensor based on dialdehyde cellulose/ionic liquid composite. Anal Biochem 471:38–43
Sung D, Yang S (2014) Facile method for constructing an effective electron transfer mediating layer using ferrocene-containing multifunctional redox copolymer. Electrochim Acta 133:40–48
Liang R, Fan L, Huang D, Qiu J (2011) A label-free amperometric immunosensor based on redox-active ferrocene-branched chitosan/multiwalled carbon nanotubes conductive composite and gold nanoparticles. Electroanal 23:719–727
Wei Z, Sun X, Li Z, Fang Y, Ren G, Huang Y, Liu J (2011) Highly sensitive deoxynivalenol immunosensor based on a glassy carbon electrode modified with a fullerene/ferrocene/ionic liquid composite. Microchim Acta 172:365–371
Qiu JD, Wang R, Liang RP, Xia XH (2009) Electrochemically deposited nanocomposite film of CS-fc/au NPs/GOx for glucose biosensor application. Biosens Bioelectron 24:2920–2925
Senel M, Nergiz C, Cevik E (2013) Novel reagentless glucose biosensor based on ferrocene cored asymmetric PAMAM dendrimers. Sensors Actuators B Chem 176:299–306
Feng T, Qiao X, Wang H, Sun Z, Hong C (2016) A sandwich-type electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of optimized ferrocene functionalized Fe3O4@SiO2 as labels. Biosens Bioelectron 79:48–54
Stobiecka M, Hepel M (2011) Effect of buried potential barrier in label-less electrochemical immunodetection of glutathione and glutathione-capped gold nanoparticles. Biosens Bioelectron 26:3524–3530
Niu Y, Yang T, Ma S, Peng F, Yi M, Wan M, Mao C, Shen J (2017) Label-free immunosensor based on hyperbranched polyester for specific detection of α-fetoprotein. Biosens Bioelectron 92:1–7
Li N, Ma H, Cao W, Wu D, Yan T, Du B, Wei Q (2015) Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens Bioelectron 74:786–791
Zhang P, Huang H, Wang N, Li H, Shen D, Ma H (2017) Duplex voltammetric immunoassay for the cancer biomarkers carcinoembryonic antigen and alpha-fetoprotein by using metal-organic framework probes and a glassy carbon electrode modified with thiolated polyaniline nanofibers. Microchim Acta 184(10):4037–4045
Jia H, Yang T, Zuo Y, Wang W, Xu J, Lu L, Li P (2017) Immunosensor for a-fetoprotein based on a glassy carbon electrode modified with electrochemically deposited N-doped graphene, gold nanoparticles and chitosan. Microchim Acta 184(10):3747–3753
Shen YM, Shen GY, Zhang YY (2018) A versatile matrix of ionic liquid functionalized with aldehyde and ferrocene for label-free electrochemical immunosensors. Anal methods, in press https://doi.org/10.1039/c8ay00108a
Shen G, Xu H, Gurung AS, Yang Y, Liu G (2013) Lateral flow immunoassay with the signal enhanced by gold nanoparticle aggregates based on polyamidoamine dendrimer. Anal Sci 29:799–804
Xu T, Chi B, Wu F, Ma S, Zhan S, Yi M, Xu H, Mao C (2017) A sensitive label-free immunosensor for detection α-fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens Bioelectron 95:87–93
Yuan Y, Li S, Xue Y, Liang T, Cui L, Li Q, Zhou S, Huang Y, Li G, Zhao Y (2017) A Fe3O4@au-based pseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal Biochem 534:56–63
Wang H, Li X, Mao K, Li Y, Du B, Zhang Y, Wei Q (2014) Electrochemical immunosensor for α-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal Biochem 465:121–126
Qi T, Liao J, Li Y, Peng J, Li W, Chu B, Li H, Wei Y, Qian Z (2014) Label-free α-fetoprotein immunosensor established by the facile synthesis of a palladium–graphene nanocomposite. Biosens Bioelectron 61:245–250
Wang L, Gan X (2009) Antibody-functionalized magnetic nanoparticles for electrochemical immunoassay of α-1-fetoprotein in human serum. Microchim Acta 164:231–237
Acknowledgments
The authors gratefully acknowledge the financial supports from Hunan Provincial Natural Science Foundation of China (2016JJ6105) and Foundation of Hunan University of Arts and Science (17ZD03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 98 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Shen, G. & Zhang, Y. Voltammetric immunoassay for α-fetoprotein by using a gold nanoparticle/dendrimer conjugate and a ferrocene derived ionic liquid. Microchim Acta 185, 346 (2018). https://doi.org/10.1007/s00604-018-2886-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2886-3